Abstract
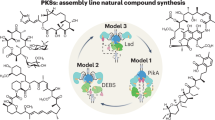
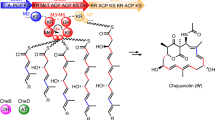
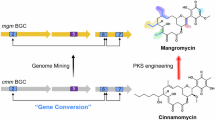
Modular polyketide synthases are multienzymes responsible for the biosynthesis of a large number of clinically important natural products. They contain multiple sets, or modules, of enzymatic activities, distributed between a few giant multienzymes and there is one module for every successive cycle of polyketide chain extension. We show here that each multi-enzyme in a typical modular polyketide synthase forms a (possibly helical) parallel dimer, and that each pair of identical modules interacts closely across the dimer interface. Such an arrangement would allow identical modules to share active sites for chain extension, and thus to function independently of flanking modules, which would have important implications both for mechanisms of evolution of polyketide synthases and for their future genetic engineering.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cortés, J., Haydock, S.F., Roberts, G.A., Bevitt, D.J. & Leadlay, P.F. An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature 348, 176–178 (1990).
Donadio, S., Staver, M.J., McAlpine, J.B., Swanson, S.J. & Kartz, L. Modular organization of genes required for complex polyketide formation. Science 252, 675–679 (1991).
MacNeil, D.J. et al. Complex organization of the Streptomyces avermitilis genes encoding the avermectin polyketide synthase. Gene 115, 119–125 (1992).
Swan, D.G., Rodríguez, A.M., Vilches, C., Méndez, C. & Salas, J.A. Characterization of a Streptomyces antibioticus gene encoding a type I polyketide synthase which has an unusual coding sequence. Mol. Gen. Genet. 242, 358–362 (1994).
Schwecke, T. et al. The biosynthetic gene cluster for the polyketide immunosuppressant rapamycin. Proc. Natl. Acad. Sci. USA 92, 7839–7843 (1995)
Hu, Z.H. et al. Repeated polyketide synthase modules involved in the biosynthesis of a heptaene macrolide by Streptomyces sp. FR-008. Mol. Microbiol. 14, 163–172 (1994).
Hopwood, D.A. & Sherman, D.H. Molecular genetics of polyketide synthesis and its comparison to fatty acid biosynthesis. Annu. Rev. Genet. 24, 37–66 (1990).
Staunton, J. The extraordinary enzymes involved in erythromycin biosynthesis. Ang. Chem. Int. Ed. Engl. 30, 1302–1306 (1991).
Katz, L. & Donadio, S. Polyketide synthesis - prospects for hybrid antibiotics. Annu. Rev. Microbiol. 47, 875–912 (1993).
Hutchinson, C.R. & Fujii, I. Polyketide synthase gene manipulation: a structure-function approach in engineering novel antibiotics. Annu. Rev. Microbiol. 49, 201–238 (1995).
Bevitt, D.J., Cortés, J., Haydock, S.F. & Leadlay, P.F. 6-deoxyerythronolide B synthase-2 from Saccharopolyspora erythraea -cloning of the structural gene, sequence analysis and inferred domain structure of the multifunctional enzyme. Eur. J. Biochem. 204, 39–49 (1992).
Wakil, S.J. Fatty acid synthase - a proficient multifunctional enzyme. Biochemistry 28, 4523–4530 (1989).
Joshi, A.K. & Smith, S. Construction, expression, and characterization of a mutated animal fatty acid synthase deficient in the dehydrase function. J. Biol. Chem. 268, 22508–22513 (1995).
Caffrey, P., Bevitt, D.J., Staunton, J. & Leadlay, P.F. Identification of DEBS-1, DEBS-2 and DEBS-3, the multienzyme polypeptides of the erythromycin-producing polyketide synthase from Saccharopolyspora erythraea. FEBS Letters 304, 225–228 (1992).
Kao, C.M., Luo, G.L., Katz, L., Cane, D.E. & Khosla, C. Engineered biosynthesis of a triketide lactone from an incomplete modular polyketide synthase. J. Amer. Chem. Soc. 116, 11612–11613 (1994).
Brown, M.J.B., Cortés, J., Cutter, A.L., Leadlay, P.F. & Staunton, J.A. mutant generated by expression of an engineered DEBS1 protein from the erythromycin-producing polyketide synthase (PKS) in Streptomyces coelicolor produces the triketide as a lactone but the major product is the nor-analogue derived from acetate as starter acid. J. C. S. Chem. Commun. 1517–1518 (1995).
Cortés, J., Wiesmann, K.E.H., Roberts, G.A., Brown, M.J.B., Staunton, J. & Leadlay, P.F. Repositioning of a domain in a modular polyketide synthase to promote specific chain cleavage. Science 268, 1487–1489 (1995).
Wiesmann, K.E.H., Cortés, J., Brown, M.J.B., Cutter, A.L., Staunton, J. & Leadlay, P.F. Polyketide synthesis in vitro on a modular polyketide synthase. Chemistry & Biology 2, 583–589 (1995).
Pieper, R., Luo, G.L., Cane, D.E. and Khosla, C. Cell-free synthesis of polyketides by recombinant polyketide synthases. Nature 378, 263–266 (1995)
Aparicio, J.F., Caffrey, P., Marsden, A.F.A., Staunton, J. & Leadlay, P.F. Limited proteolysis and active site studies of the first multienzyme component of the erythromycin-producing polyketide synthase. J. Biol. Chem. 269, 8524–8528 (1994).
Roberts, G.A., Staunton, J. & Leadlay, P.F. Heterologous expression in Escherichia coli of an intact multienzyme component of the erythromycin-producing polyketide synthase. Eur. J. Biochem. 214, 305–311 (1994).
Marsden, A.F.A., Caffrey, P., Aparicio, J.F., Loughran, M.S., Staunton, J. & Leadlay, P.F. Stereospecific acyl transfers on the erythromycin-producing polyketide synthase. Science 263, 378–380 (1994).
Aggarwal, R., Caffrey, P., Leadlay, P.F., Smith, C.J. & Staunton, J. The thioesterase of the erythromycin-producing polyketide synthase -mechanistic studies in vitro to investigate its mode of action and substrate specificity. J. C. S. Chem. Commun. 1519–1520 (1995).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Durchschlag, H. In: Thermodynamic Data for Biochemistry and Biotechnology (ed. Hinz, H.-J.) 45–128. (Springer Verlag, Berlin, 1986).
Laue, T.M., Shah, B.D., Ridgeway, T.M. & Pelletier, S.L. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science (eds. Harding, S.E., Rowe, A.J. & Norton, J.C.) 90–125. Royal Society of Chemistry, Cambridge.
Matsudaira, P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J. Biol. Chem. 262, 10035–10038 (1987).
Donadio, S. & Katz, L. Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin biosynthesis. Gene 111, 51–60 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Staunton, J., Caffrey, P., Aparicio, J. et al. Evidence for a double-helical structure for modular polyketide synthases. Nat Struct Mol Biol 3, 188–192 (1996). https://doi.org/10.1038/nsb0296-188
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nsb0296-188
This article is cited by
-
Enzymology of assembly line synthesis by modular polyketide synthases
Nature Chemical Biology (2023)
-
Synthetic biology of polyketide synthases
Journal of Industrial Microbiology and Biotechnology (2018)
-
Harnessing natural product assembly lines: structure, promiscuity, and engineering
Journal of Industrial Microbiology and Biotechnology (2016)
-
The structural biology of biosynthetic megaenzymes
Nature Chemical Biology (2015)
-
Structure of a modular polyketide synthase
Nature (2014)