Abstract
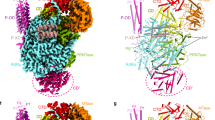
Bovine pancreatic ribonuclease (RNase A) forms two types of dimers (a major and a minor component) upon concentration in mild acid. These two dimers exhibit different biophysical and biochemical properties. Earlier we reported that the minor dimer forms by swapping its N-terminal α-helix with that of an identical molecule. Here we find that the major dimer forms by swapping its C-terminal β-strand, thus revealing the first example of three-dimensional (3D) domain swapping taking place in different parts of the same protein. This feature permits RNase A to form tightly bonded higher oligomers. The hinge loop of the major dimer, connecting the swapped β-strand to the protein core, resembles a short segment of the polar zipper proposed by Perutz and suggests a model for aggregate formation by 3D domain swapping with a polar zipper.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
References
Bennett, M. J., Choe, S. & Eisenberg, D. S. Proc. Natl. Acad. Sci. USA 91, 3127–3131 (1994).
Crestfield, A. M., Stein, W. H. & Moore, S. Arch. Biochem. Biophys. Suppl. 1, 217–222 (1962).
Liu, Y., Hart, P. J., Schlunegger, M. P. & Eisenberg, D. S. Proc. Natl. Acad. Sci. USA 95, 3437–3442 (1998).
Fruchter, R. C. & Crestfield, A. M. J. Biol. Chem. 240, 3868–3874 (1965).
Gotte, G., Bertoldi, M. & Libonati, M. Eur. J. Biochem. 265, 680–687 (1999).
Libonati, M., Bertoldi, M. & Sorrentino, S. Biochem. J. 318, 287–290 (1996).
Lisgarten, J. N., Maes, D., Wyns, L., Aguilar, C. F. & Palmer, R. A. Acta Crystallogr. D 51, 767–771 (1995).
Schreier, A. A. & Baldwin, R. L. J. Mol. Biol. 105, 409–426 (1976).
Haber, E. & Anfinsen, C. B. J. Biol. Chem. 236, 422–426 (1961).
Kato, I. & Anfinsen, C. B. J. Biol. Chem. 244, 1004–1007 (1969).
Bennett, M. J., Schlunegger, M. P. & Eisenberg, D. S. Protein Sci. 4, 2455–2468 (1995).
Klafki H. W. et al. Biol. Chem. Hoppe-Seyler 374, 1117–1122 (1993).
Cohen, F. E. & Prusiner, S. B. Annu. Rev. Biochem. 67, 793–819 (1998).
Schlunegger, M. P., Bennett, M. J. & Eisenberg, D. S. In Advances in protein chemistry (eds Richards, F. M. Eisenberg, D. S. & Kim P. S.) 50, 61–122 (Academic Press, New York; 1997).
Chiti F. et al. EMBO J. 19, 1441–1449 (2000).
Perutz, M. F., Johnson, T., Suzuki, M. & Finch, J. T. Proc. Natl. Acad. Sci. USA 91, 5355–5358 (1994).
Perutz, M. F. Trends Biochem. Sci. 24, 58–63 (1999).
Scherzinger E. et al. Cell 90, 549–558 (1997).
Maddelein, M. L. & Wickner, R. B. Mol. Cell. Biol. 19, 4516–4524 (1999).
Fink, A. L. Folding & Design 3, R9–R23 (1998).
Sipe, J. D. Crit. Rev. Clin. Lab. Sci. 31, 325–354 (1994).
Bunn, C. W. Chemical crystallography. 278–280 (The Clarendon Press, Oxford, UK; 1952).
Glover, J. R. et al. Cell 89, 811–819 (1997).
Satyal, S. H. et al. Proc. Natl. Acad. Sci. USA 97, 5750–5755 (2000).
Navaza, J. Acta Crystallogr. A 50, 157–163 (1994).
Birdsall, D. L. & McPherson, A. J. Biol. Chem. 267, 22230–22236 (1992).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta Crystallogr. A 47, 110–119 (1991).
Brunger A. T. et al. Acta Crystallogr. D 54, 905–921 (1998).
Merritt, E. A. & Bacon, D. J. Methods Enzymol. 277, 505–524 (1997).
Evans, S. E. J. Mol. Graphics 11, 134–138 (1993).
Acknowledgements
We thank D. Cascio, O. Dym, D. Anderson and M. Sawaya for technical advice, I. Xenario, M. J. Bennett and G. Kleiger for useful discussion, and A. McPherson for generously providing coordinates of RNase A and d(pA)4 complex. This work was supported by NSF, NIH, DOE, and Italian MURST-Prin 1999.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Gotte, G., Libonati, M. et al. A domain-swapped RNase A dimer with implications for amyloid formation. Nat Struct Mol Biol 8, 211–214 (2001). https://doi.org/10.1038/84941
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/84941
This article is cited by
-
Breakdown of supersaturation barrier links protein folding to amyloid formation
Communications Biology (2021)
-
Cytotoxic Potential of Novel Bacillary Ribonucleases Balnase and Balifase
BioNanoScience (2020)
-
A five-residue motif for the design of domain swapping in proteins
Nature Communications (2019)
-
Probing Medin Monomer Structure and its Amyloid Nucleation Using 13C-Direct Detection NMR in Combination with Structural Bioinformatics
Scientific Reports (2017)
-
Linking in domain-swapped protein dimers
Scientific Reports (2016)