Abstract
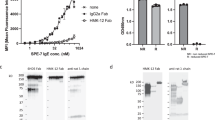
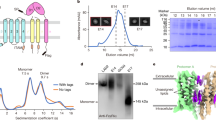
We have designed synthetic peptide inhibitors of the interaction between IgE and its high affinity receptor, FcεRI. The structure of the second domain of CD2 was used as a modelling template for the second α-chain domain of FcεRI, the C-C′ loop of which has been implicated in the interaction with IgE. An L-amino acid peptide and a retro-enantiomeric D-amino acid peptide were designed to mimic the conformation of the C-C′ region. Both peptides were cyclized by disulphide bond formation between terminal cysteine residues, and show mirror image symmetry by circular dichroism analysis. The C-C′ peptide mimics act as competitive inhibitors of lgE binding. The cyclic L- and retro D-peptides exhibited KDs of approximately 3 μM and 11 μM, respectively, for IgE. Further, the peptides inhibit IgE-mediated mast cell degranulation, an in vitro model of an allergic response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Banner, D.W. & Hadvary, P. Crystallographic analysis at 3.0-Å resolution of the binding to human thrombin of four active site-directed inhibitors. J. Biol. Chem. 266, 20085–20093 (1991).
Navia, M.A. & Murcko, M.A. Use of structural information in drug design. Curr. Opin. Stuct. Biol. 2, 202–210 (1992).
Chen, S. et al. Design and synthesis of a CD4 3-turn mimetic that inhibits human immunodeficiency virus envelope glycoprotein gpl2O binding and infection of human lymphocytes. Proc. Natl. Acad. Sci. USA 89, 5872–5876 (1992).
Jameson, B.A., McDonnell, J.M., Marini, J.C. & Korngold, R. A rationally designed CD4 analogue inhibits experimental allergic encephalomyelitis. Nature 368, 744–746 (1994).
Nakanishi, H. et al. Peptide mimetics ofthe thrombin-bound structure of fibrinopeptide A. Proc. Natl. Acad. Sci. USA 89, 1705–1709 (1992).
Sato, M. et al. M. Design, synthesis and conformational analysis of yturn peptide mimetics of bradykinin. Biochem. Biophys. Res. Comm. 187, 999–1006 (1992).
DeGrado, W.F. Design of peptides and proteins. Adv. Prot. Chem. 39, 51–124 (1998).
Ajo, D., Granozzi, G., Tondello, E. & Del Prada, A. Conformational flexibility of peptides containing αβ-unsaturated amino acid residues. I. Conformational analysis of N-acetyl-N′-methylamides of dehydroalanine and N-methyldehydroalanine. Biopolymers 19, 469–475 (1980).
Rose, G.D., Gierasch, L.M. & Smith, J.A. Turns in peptides and proteins. Adv. Prot. Chem. 37, 1–109 (1985).
Rizo, J. & Gierasch, L.M. Constrained peptides - models of bioactive peptides and protein substructures. Ann. Rev. Biochem. 61, 387–418 (1992).
Miklavc, A., Kocjan, D., Avbelj, F. & Hadzi, D. in QSAR in Drug Design and Toxicology, (eds D. Hadzi & B. Jerman-Blazic) 185–190. (Elsevier Science Publishers B.V., Amsterdam, 1987).
Riske, F. et al. High affinity human IgE receptor (FceRl). Analysis of functional domains of the α-subunit with monoclonal antibodies. J. Biol. Chem. 266, 11245–11251 (1991).
Robertson, M.W. Phage and Escherichia coli expression ofthe human high affinity immunoglobulin E receptor α-subunit ectodomain. J. Biol. Chem. 268, 12736–12743 (1993).
Mallamaci, M.A. et al. Identification of sites on the human FcεRlα subunit which are involved in binding to human and rat IgE. J. Biol. Chem. 268, 22076–22083 (1993).
Hulett, M.D., McKenzie, I.F.C. & Hogarth, P.M. Chimeric Fc receptors identify immunoglobulin-binding regions in human FcγRII and FcεRI. Eur. J. Immunol. 23, 640–645 (1993).
Scarselli, E., Esposito, G. & Traboni, C. Receptor phage: Display of functional domains of the high affinity IgE receptor on the M13 phage surface. FEBS Lett. 329, 223–226 (1993).
Shimizu, A., Tepler, I., Bemfrey, P.N., Berenstein, E.H & Leder, R. Human and rat mast-cell high-affinity immunoglobulin-E receptors - characterization of putative alpha-chain gene products. Proc. Natl. Acad. Sci. USA. 85, 1907–1911 (1988).
Sutton, B.J. & Gould, H.L. The human IgE network. Nature. 366, 421–428 (1993).
Hogarth P.M. et al. Identification of the immunoglobulin binding regions of Fc-gamma-RII and Fc-epsilon-RI. Immunol. Rev. 125, 21–35 (1992).
Hibbs, M.L., Tolvanen, M. & Carpen, O. Membrane-proximal Ig-like domain of FcγRII (CD16) contains residues critical for ligand binding. J. Immunol. 152, 4466–4474 (1994).
Hulett, M.D., Witort, E., Brinkworth, R.I., McKenzie, I.F.C. & Hogarth, P.M. Identification of the lgG binding site of the human low affinity receptorfor lgG FcγRII. J. Biol. Chem. 269, 15287–15293 (1994).
Williams, A.F. & Barclay, A.N. The immunoglobulin superfamily - domains for cell surface recognition. Ann. Rev. Immunol. 6, 381–405 (1988).
Ryu, S.E. et al. Crystal structure of an HIV-binding recombinant fragment of human CD4. Nature. 348, 419–426 (1990).
Wang, J. et al. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature 348, 411–418 (1990).
Brady, R.L. et al. Crystal structure of domains 3 and 4 of rat CD4: Relation to the NH2-terminal domains. Nature 260, 979–983 (1993).
Jones, E.Y., Davis, S.J., Williams A.F., Harlos, K. & Stuart, D.I. Crystal structure at 2.8 Å resolution of a soluble form of the cell adhesion molecule CD2. Nature 360, 232–239 (1992).
Padlan, E.A. & Helm, B.A. A modeling study of the α-subunit of human high-affinity receptor for immunoglobulin-E. Receptor 2, 129–143 (1992).
Bork, P., Holm, L. & Sander, C. The immunoglobulin fold: structural classification, sequence patterns and common core. J. Molec. Biol. 242, 309–320 (1994).
Laskowski, R.A., MacArthur, M.W., Moss, D.S & Thornton, J.M. PROCHECK - A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 (1993).
Jackson, S. et al. Template-constrained cyclic peptides: Design of high affinity ligands for GPIIb/Illa. J. Amer. Chem. Soc. 116, 3220–3230 (1994).
Guichard, G. et al. Antigenic mimicry of natural L-peptideswith retroinverso-peptidomimetics. Proc. Natl. Acad. Sci. USA 91, 9765–9769 (1994).
Dintzis, H.M., Symer, D.E., Dintzis, R.Z., Zawadzke, L.E. & Berg, J.M. A comparison of immunogenicity of a pair of enantiomeric proteins. Proteins 16, 306–308 (1993).
Goodman, M. & Chorev, M. On the concept of linear modified retro peptide structures. Acc. Chem. Res. 12, 1–7 (1979).
Guptsarma, P. Reversal of peptide backbone may result in the mirroring of protein structure.FEBS Lett. 310, 205–210 (1992).
Shemyakin, M.M., Ovchinnikov, Yu.A. & Ivanov, V.T. Topochemical investigations on peptide systems. Angew. Chem. Int. Ed. Engl. 8, 492–499 (1969).
Chorev, M., Willson, G.C. & Goodman, M. A general approach to retroisomeric linear peptide synthesis. J. Amer. Chem. Soc. 99, 8075–8076 (1977).
Bush, C.A., Sarkar, S.K. & Kopple, K.D. Circular dichroism of turns in peptides and proteins. Biochemistry 17, 491–494 (1978).
Gierasch, L.M., Deber, C.M., Madison, V., Niu, C.H. & Blout, E.R. Conformations of (X-L-Pro-Y)2 cyclic hexapeptides. Preferred β-turn conformers and implications for β turns in proteins. Biochemistry 20, 4730–4738 (1981).
Woody, R.W. Aromatic side-chain contributions to the far ultraviolet circular dichroism of peptides and proteins. Biopolymers 17, 1451–1467 (1978).
Hider, R.C., Drake, A.F. & Tamiya, N. An analysis of the 225-230-nm CD band of elapid toxins. Biopolymers 27, 113–122 (1987).
St. Pierre, S., Ingwall, R.T., Verlander, M.S. & Goodman, M. Conformational studies of sequential polypeptides containing lysine and tyrosine. Biopolymers 17, 1837–1848 (1978).
Chang, C.T., Wu, C-S.C. & Yang, J.T. Circular dichroic analysis of protein conformations: inclusion of the β-turns. Analyt. Biochem. 91, 13–31 (1978).
Jonsson, U. et al. Real-time biospecific interaction analysis using surface plasmon resonance and a sensor chip technology. BioTechniques 11, 620–627 (1991).
Karlsson, R., Michaelsson, A. & Mattsson, L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145, 229–240 (1991).
Metzger, H. et al. The receptor with high affinity for immunoglobulin-E. Ann. Rev. Immunol. 4, 419–470 (1986).
Hulett, M.D. & Hogarth, P.M. Molecular basis of Fc receptor function. Adv. Immunol. 57, 1–127 (1994).
Hakimi, J. et al. The α subunit of the human IgE receptor (FcεRI) is sufficient for high affinity IgE binding. J. Biol. Chem. 265, 22079–22081 (1990).
MacGlashan, D.W., Jr., Peters, S.P., Warner, J. & Lichtenstein, L.M. Characteristics of human basophil sulfopeptide leukotriene release:releasability defined as the ability of the basophil to respond to dimeric cross-links. J. Immunol. 136, 2231–2239 (1986).
Ra, C. et al. Soluble human high-affinity receptor for IgE abrogates the gE-mediated allergic reaction. Int. Immunol. 5, 47–54 (1993).
Clackson, T. & Wells, J.A. A hot spot of binding energy in a hormone receptor interface. Science 267, 383–386 (1995).
McDonnell, J.M., Blank, K.J., Rao, P.E. & Jameson, B.A. Direct involvement of the CDR3-like domain of CD4 in T helper cell activation. J. Immunol. 149, 1626–1630 (1992).
Glocker, M.O., Borchers, C., Fiedler, W., Suckau, D. & Przybylski, M. Molecular characterization of surface topology in protein tertiary structure by amino-acylation and mass spectrometric peptide mapping. Bioconjugate Chem. 5, 83–90 (1994).
Karlsson, R. Real-time competitive kinetic analysis of interactions between low-molecular-weight ligands in solution and surface- immobilized receptors. Analyt. Biochem. 221, 142–151 (1994).
Schwartzbaum, S. et al. Mapping of murine IgE epitopes involved in lgE-Fcε receptor interactions. Eur. J. Immunol. 19, 1015–1023 (1989).
Evans, S.V. SETOR: hardware lighted three dimensional solid model representations of macromolecules. J. Mol. Graphics 11, 134–138 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
McDonnell, J., Beavil, A., Mackay, G. et al. Structure based design and characterization of peptides that inhibit IgE binding to its high-affinity receptor. Nat Struct Mol Biol 3, 419–426 (1996). https://doi.org/10.1038/nsb0596-419
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nsb0596-419
This article is cited by
-
An allosteric interleukin-1 receptor modulator mitigates inflammation and photoreceptor toxicity in a model of retinal degeneration
Journal of Neuroinflammation (2020)
-
IgE and mast cells in allergic disease
Nature Medicine (2012)
-
Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcɛRI
Nature Structural & Molecular Biology (2011)
-
Structure–based design and characterization of exocyclic peptidomimetics that inhibit TNFα binding to its receptor
Nature Biotechnology (1997)