Abstract
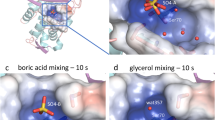
The structure of the plasmid-mediated β-lactamase TEM-1 has been solved in complex with a designed boronic acid inhibitor (1R)-1-acetamido-2-(3-carboxyphenyl)ethane boronic acid at 1.7 Å resolution. The boronate inhibitor was designed based on the crystallographic coordinates of the acyl-enzyme intermediate of TEM-1 bound to the substrate penicillin G. The boronate–TEM-1 complex is highly ordered and defines a novel transition state analogue of the deacylation step in the β-lactamase reaction pathway. The design principles of this highly effective inhibitor (Ki=110 nM) and the resulting structural and mechanistic implications are presented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Abraham, E.P. & Chain, E.B. An enzyme from bacteria able to destroy penicillin. Nature 146, 837 (1940).
Frere, J.M. β-lactamases and bacterial resistance to antibiotics. Molec. Microbiol. 16, 385–395 (1995).
Bush, K., Jacoby, G.A. & Medeiros, A.A. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob. Agents Chemother. 39, 1211–1233 (1995).
Blazquez, J., Morosini, M.I., Negri, M.C., Gonzalez-Leiza, M. & Baquero, F. Single amino acid replacements at positions altered in naturally occurring extended-spectrum TEM β-lactamases. Antimicrob. Agents Chemother. 39, 145–149 (1995).
Urban, C., Meyer, K.S., Mariano, N., Rahal, J.J., Flamm, R., Rasmussen, B.A. & Bush, K. Identification of TEM-26 β-lactamase responsible for a major outbreak of ceftazidime-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 38, 392–395 (1994).
Rasmussen, B.A., Bradford, P.A., Quinn, J.P., Wiener, J., Weinstein, R.A. & Bush, K. Genetically diverse ceftazidime-resistant isolates from a single center: biochemical and genetic characterization of TEM-10 β-lactamases encoded. Antimicrob. Agents Chemother. 37, 1989–1992 (1993).
Quinn, J.P., Miyashiro, D., Sahm, D., Flamm, R. & Bush, K. Novel plasmid-mediated β-lactamase (TEM-10) conferring selective resistance to ceftazidime and aztreonam in clinical isolates of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 33, 1451–1456 (1989).
Gutmann, L., Kitzis, M.D. & Aear, J.F. Evolution of enzymatic mechanisms of resistance amongst β-lactam antibiotics. J. Int. Med. Res. 18, 371–471 (1990).
Jacoby, G.A. & Mederios, A.A. More extended spectrum β-lactamases. Antimicrob. Agents Chemother. 35, 1697–1700 (1991).
Sougakoff, W., Goussard, S., Gerbaud, G. & Courvalin, P. Plasmid-mediated resistance to third generation cephalosporins due to point-mutations in TEM-type penicillinase genes. Rev. Infect. Dis. 10, 879–884 (1988).
Barthelemy, M., Peduzzi, J., Ben Yaghlane, H. & Labia, R. Single amino acid substitutions between SHV-1 β-lactamase and cefotaxime- hydrolyzing SHV-2 enzyme. FEBS Lett. 234, 217–220 (1988).
Reading, C., Farmer, T. & Cole, M. The β-lactamase stability of amoxycillin with the β-lactamase inhibitor, clavulanic acid. J. Antimicrob. Chemother. 11, 27–32 (1983).
Reading, C. & Cole, M. Clavulanic acid: a β-lactamase-inhibiting β-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 11, 852–857 (1977).
Bush, K., Macalintal, C., Rasmussen, B.A., Lee, V.J. & Yang, Y. Kinetic interactions of tazobactam with β-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37, 851–858 (1993).
Blazquez, J., Baquero, M.F., Canton, R., Alos, R. & Baquero, F. Characterization of a new TEM-type β-lactamase resistant to clavulanate, sublactam, and tazobactam in a clinical isolate of Escherichia coli. Antimicrob. Agents Chemother. 37, 2059–2067 (1993).
Leleu, G., Kitzis, M.D., Vallois, J.M., Gutmann, L. & Decazes, J.M. Different ratios of the piperacillin-tazobactam combination for treatment of experimental meningitis due to Klebsiella pneumoniae producing the TEM-3 extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 38, 195–199 (1994).
Bush, K., Flamm, R.K., Ohringer, S., Singer, S.B., Summerill, R. & Bonner, D.P. Effect of clavulanic acid on activity of β-lactam antibiotics in Serratia marcescens isolates producing both a TEM β-lactamase and a chromosomal cephalosporinase. Antimicrob. Agents Chemother. 35, 2203–2208 (1991).
Strynadka, N.C. et al. Molecular structure of the acyl-enzyme intermediate in β-lactam hydrolysis at 1.7 Å resolution. Nature 359, 700–705 (1992).
Matagne, A. & Frere, J.M. Contribution of mutant analysis to the understanding of enzyme catalysis: the case of class A β-lactamases. Biochem. Biophys. Acta 1246, 109–127 (1995).
Herzberg, O. & Moult, J. Penicillin-binding and degrading enzymes. Curr. Opin. Struct. Biol. 1, 946–952 (1991).
Pratt, R.F. Inhibition of a class C β-lactamase by a specific phosphonate monoester. Science 246, 917–919 (1989).
Pratt, R.F. & Rahil, J. Kinetics and mechanism of β-lactamase inhibition by phosphonamidates: the quest for a proton. Biochemistry 32, 10763–10772 (1993).
Pratt, R.F. & Rahil, J. Phosphonate monoester inhibitors of class A β-lactamases. Biochem. J. 275, 793–795 (1991).
Chen, C.C.H., Rahil, J., Pratt, R.F. & Herzberg, O. Structure of a phosphonate-inhibited β-lactamase. An analog of the tetrahedral transition state intermediate of β-lactam hydrolysis. J. Mol. Biol. 234, 165–179 (1993).
Rahil, J. & Pratt, R.F. Characterization of covalently bound enzyme inhibitors as transition-state analogs by protein stability measurements: phosphonate monoester inhibitors of a β-lactamase. Biochemistry 33, 116–125 (1994).
Dobozy, O., Mile, I., Ferencz, I. & Csanyi, V. Effect of electrolytes on the activity and iodine sensitivity of penicillinase from B. cereus. Acta Biochim. Biophys. Acad. Sci. Hung. 6, 97–105 (1971)
Crompton, I.E., Cuthbert, B.K., Lowe, G. & Waley, S.G. β-lactamase inhibitors. The inhibition of serine β-lactamases by specific boronic acids. Biochem. J. 251, 453–459 (1988).
Beeseley, T. et al. The inhibition of class C β-lactamases by boronic acids. Biochem. J. 209, 229–233 (1983).
Baldwin, J.E. et al. Direct observation of a tetrahedral boronic acid-β-lactamase complex using boron-11 NMR spectroscopy. J. Chem. Soc. Chem. Commun. 121, 573–574 (1991).
Martin, R. & Jones, J.B. Rational design and synthesis of a highly effective transition state analog inhibitor of the RTEM-1 β-lactamase. Tetrahedron Lett. 36, 8394–8402 (1995).
Ambler, R.P. et al. A standard numbering scheme for the class A β-lactamases. Biochem. J. 276, 269–270 (1991).
Knowles, J.R. Penicillin resistance: the chemistry of β-lactamase inhibition. Acct. Chem. Res. 18, 97–104 (1985).
Herzberg, O. & Moult, J. Bacterial resistance to β-lactam antibiotics: crystal structure of β-lactamase from Staphylococcal aureus PC1 at 2.5 resolution. Science 236, 694–701 (1987).
Sutton, B.J. et al. An X-ray-crystallographic study of β-lactamase II from Bacillus cereus at 0.35 nm resolution. Biochem. J. 248, 181–188 (1987).
Herzberg, O.J. Refined crystal structure of β-lactamase from Staphylococcus aureus PC1 at 2.0 Å resolution. J. Mol. Biol. 217, 701–719 (1991).
Jelsch, C., Lenfant, F., Masson, J.M. & Samama, J.P. β-lactamase TEM-1 of E. coli. Crystal structure determination at 2.5 Å resolution. FEBS Lett. 299, 135–142 (1992).
Jelsch, C., Mourey, L., Masson, J.M. & Samama, J.P. Crystal structure of Escherichia coli TEM1 β-lactamase at 1.8 Å resolution. Proteins 16, 364–383 (1993).
Knox, J.R. & Moews, P.C. β-lactamase of Bacillus licheniformis 749/C. Refinement at 2 Å resolution and analysis of hydration. J. Mol. Biol. 220, 435–455 (1991).
Adachi, H., Ohta, T. & Matsuzawa, H. Site-directed mutants at position 166 of RTEM-1 β-lactamase that form a stable acyl-enzyme intermediate with penicillin. J. Biol. Chem. 266, 3186–3191 (1991).
Lobkovsky, E. et al. Evolution of an enzyme activity: crystallographic structure at 2 Å resolution of cephalosporinase from the ampC gene of Enterobacter cloacae P99 and comparison with a class A penicillinase. Proc. Natl. Acad. Sci. USA 90, 11257–11261 (1993).
Swaren, P. et al. Electrostatic analysis of TEM1 β-lactamase: effect of substrate binding, steep potential gradients and consequences of site-directed mutations. Structure 3, 603–614 (1995).
Martin, R., Gold, M. & Jones, J.B. Inhibition of the RTEM-1 β-lactamase by boronic acids. Bioorg. Med. Chem. Lett. 4, 1229–1234 (1994).
Leung, Y.C., Robinson, C.V., Aplin, R.T. & Waley, S.G. Site-directed mutagenesis of β-lactameas I: role of Glu-166. Biochem. J. 299, 671–678 (1994).
Christiansen, H., Martin, M.T. & Waley, S.G. β-lactamases as fully efficient enzymes. Determination of all the rate constants in the acyl-enzyme mechanism. Biochem. J. 266, 853–861 (1990).
Aplin, R.T., Baldwin, J.E., Schofield, C.J. & Waley, S.G. Use of electrospray mass spectrometry to directly observe an acyl enzyme intermediate in β-lactamase catalysis. FEBS Lett. 277, 212–214 (1990).
Little, C., Emanuel, E.L., Gagnon, J. & Waley, S.G. Identification of an essential glutamic acid residue in β-lactamase II from Bacillus cereus. Biochem. J. 233, 465–469 (1986).
Escobar, W.A., Tan, A.K. & Fink, A.L. Site-directed mutagenesis of β-lactamase leading to accumulation of a catalytic intermediate. Biochemistry 30, 10783–10787 (1991).
Gibson, R.M., Christensen, H. & Waley, S.G. Site-directed mutagenesis of β-lactamase I. Single and double mutants of Glu-166 and Lys-73. Biochem. J. 272, 613–619 (1990).
Viera, J. & Messing, J. Production of single-stranded plasmid DNA. Meth. Enzym. 153, 3–11 (1987).
Xuong, N.H., Sullivan, D., Nielson, C., Hamlin, R. & Anderson, D. Strategy for data collection from protein crystals using a multiwire counter area detector diffractometer. J. Appl. Crystallogr. 18, 342–350 (1985).
Howard, A.J., Nielson, C. & Xuong, N.H. Software for a diffractometer with multiwire area detector. Meth. Enzymol. 114, 452–472 (1985).
Tronrud, D.E. Conjugate-direction minimization: an improved method for the refinement of macromolecules. Acta Crystallogr. A48, 912–916 (1992).
Chen, C.C.H. & Herzberg, O. Inhibition of β-lactamase by clavulanate: trapped intermediates in cryocrystallographic studies. J. Mol. Biol. 224, 1103–1113 (1992).
Bernstein, F.C. et al. The Protein Data Bank: A computer-based archival filefor macromolecularstructures. J. Mol. Biol. 112, 535–542 (1977).
Merrit, E.A. & Murphy, M.E.P. Raster3D Version 2.0 a program fro photorealistic molecular graphics. Acta Crystallgr. D50, 869–873 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Strynadka, N., Martin, R., Jensen, S. et al. Structure-based design of a potent transition state analogue for TEM-1 β-lactamase. Nat Struct Mol Biol 3, 688–695 (1996). https://doi.org/10.1038/nsb0896-688
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/nsb0896-688
This article is cited by
-
Structural basis to repurpose boron-based proteasome inhibitors Bortezomib and Ixazomib as β-lactamase inhibitors
Scientific Reports (2022)
-
The hydrolytic water molecule of Class A β-lactamase relies on the acyl-enzyme intermediate ES* for proper coordination and catalysis
Scientific Reports (2020)
-
Search for non-lactam inhibitors of mtb β-lactamase led to its open shape in apo state: new concept for antibiotic design
Scientific Reports (2017)
-
Dynamical Aspects of TEM-1 β-Lactamase Probed by Molecular Dynamics
Journal of Computer-Aided Molecular Design (2005)