Abstract
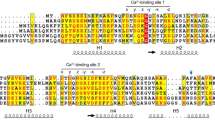
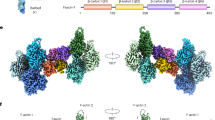
Using a new procedure that combines electron-density correlation with biochemical information, we have fitted the crystal structure of the N-terminal actin-binding domain of human T-fimbrin to helical reconstructions of fimbrin-decorated actin filaments. The map locates the N-terminal calcium-binding domain and identifies actin-binding site residues on the two calponin-homology domains of fimbrin. Based on this map, we propose a model of a fimbrin crosslink in an actin bundle and its regulation by calcium.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Castresana, J. & Saraste, M. FEBS Letters 374, 149–151. (1995).
Tilney, M.S. et al. J. Cell Biol. 109, 1711– 1723 (1989).
Matsudaira, P.T. & Burgess, D.R. J. Cell Biol. 83, 667–673 ( 1979).
Glenney, J.R., Kaulfus, P., Matsudaira, P. & Weber, K. J. Biol. Chem. 256, 9283–9288 (1981).
Bretscher, A. & Weber, K. J. Cell Biol. 86, 335–340 (1980).
de Arruda, M.V., Watson, S., Lin, C.S., Leavitt, J. & Matsudaira, P. J. Cell Biol. 111, 1069– 1079 (1990).
Yang, Y. et al. Cell 86, 655–665 (1996).
Svitkina, T.M., Verkhovsky, A.B. & Borisy, G.G. J. Cell Biol. 135, 991– 1007 (1996).
Brill, S. et al. Mol. Cell Biol. 16, 4869– 4878 (1996).
Bustelo, X.R., Ledbetter, J.A. & Barbacid, M. Nature 356, 68– 71 (1992).
Namba, Y., Ito, M., Zu, Y., Shigesada, K. & Maruyama, K. J Biochem. (Tokyo) 112, 503– 507 (1992).
Lin, C.S., Shen, W., Chen, Z.P., Tu, Y.H. & Matsudaira, P. Mol Cell Biol 14, 2457– 2467 (1994).
Goldsmith, S.C., Pokala, N., Shen, W., Fedorov, A.A., Matsudaira, P. & Almo, S.C. Nature Struct. Biol. 4, 708–712 (1997).
Hanein, D., Matsudaira, P. & DeRosier, D.J. J. Cell Biol. 139 (2), 387– 396 (1997).
Holtzman, D.A., Wertman, K.F. & Drubin, D.G. J. Cell Biol. 126, 423– 432 (1994).
Honts, J.E., Sandrock, T.S., Brower, S.M., O'Dell, J.L. & Adams, A.E. J. Cell Biol. 126, 413–422 (1994).
Bresnick, A.R., Janmey, P.A. & Condeelis, J. J. Biol. Chem. 266, 12989– 12993 (1991).
Levine, B.A., Moir, A.J., Patchell, V.B. & Perry, S.V. FEBS Lett. 298, 44–48 ( 1992).
Lebart, M.C., Mejean, C., Roustan, C. & Benyamin, Y. J. Biol. Chem. 268, 5642–5648 ( 1993).
Corrado, K., Mills, P.L. & Chamberlain, J.S. FEBS Lett 344, 255– 260 (1994).
Brower, S.M., Honts, J.E. & Adams, A.E. Genetics 140, 91– 101 (1995).
Way, M., Pope, B. & Weeds, A.G. J. Cell Biol. 119, 835– 842 (1992).
Bashour, A.M., Fullerton, A.T., Hart, M.J. & Bloom, G.S. J. Cell Biol. 137, 1555–1566 (1997).
Winder, S. et al. J. Cell Sci. 108, 63– 71 (1995).
Carugo, K.D., Banuelos, S. & Saraste, M. Nature Struct. Biol. 4, 175– 179 (1997).
Winder, S.J., Sutherland, C. & Walsh, M.P. Adv. Exp. Med. Biol. 304, 37 –51 (1991).
Hodgkinson, J.L., el-Mezgueldi, M., Craig, R ., Vibert, P ., Marston, S.B. & Lehman, W. J. Mol. Biol. 273, 150– 159 (1997).
Matsudaira, P., Mandelkow, E., Renner, W., Hesterberg, L.K. & Weber, K. Nature 301, 209–214 (1983).
Ikura, M. Trends Biochem Sci 21, 14–17 (1996).
Jones, T.A., Zou, J.-Y., Cowan, S.W. & Kjeldgaard, M. Acta Crystallogr. A47, 110–119 ( 1991).
Sayle, R.A. & Milner-White, E.J. Trends Biochem Sci 20, 374 (1995).
Lorenz, M., Popp, D. & Holmes, K.C. J. Mol. Biol. 234, 826 –836 (1993).
Kuboniwa, H., Tjandra, N., Grzesiek, S., Ren, H., Klee, C.B. & Bax, A. Nature Struct. Biol. 2, 768–776 (1995).
Acknowledgements
D.H. and N.V. wish to thank A. Brilliant for his valuable contributions to the graphics displayed in this paper. This work was supported by National Institutes of Health grants to D.DeR., W. L., R. C. (F. S. Fay), S.A. and P.M.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hanein, D., Volkmann, N., Goldsmith, S. et al. An atomic model of fimbrin binding to F-actin and its implications for filament crosslinking and regulation. Nat Struct Mol Biol 5, 787–792 (1998). https://doi.org/10.1038/1828
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1038/1828
This article is cited by
-
The Dictyostelium discoideum FimA protein, unlike yeast and plant fimbrins, is regulated by calcium similar to mammalian plastins
Scientific Reports (2023)
-
The Calcium-Dependent Switch Helix of L-Plastin Regulates Actin Bundling
Scientific Reports (2017)
-
Fimbrin phosphorylation by metaphase Cdk1 regulates actin cable dynamics in budding yeast
Nature Communications (2016)
-
Structure and dynamics of the actin-based smooth muscle contractile and cytoskeletal apparatus
Journal of Muscle Research and Cell Motility (2012)
-
Regulation of actin cytoskeleton dynamics in cells
Molecules and Cells (2010)