Abstract
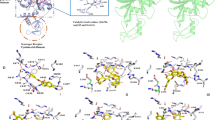
Using energy and density guided Rosetta refinement to improve molecular replacement, we determined the crystal structure of the protease encoded by xenotropic murine leukemia virus–related virus (XMRV). Despite overall similarity of XMRV protease to other retropepsins, the topology of its dimer interface more closely resembles those of the monomeric, pepsin-like enzymes. Thus, XMRV protease may represent a distinct branch of the aspartic protease family.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schlaberg, R., Choe, D.J., Brown, K.R., Thaker, H.M. & Singh, I.R. Proc. Natl. Acad. Sci. USA 106, 16351–16356 (2009).
Lombardi, V.C. et al. Science 326, 585–589 (2009).
Groom, H.C. et al. Retrovirology 7, 10 (2010).
Singh, I.R., Gorzynski, J.E., Drobysheva, D., Bassit, L. & Schinazi, R.F. PLoS ONE 5, e9948 (2010).
Wlodawer, A. & Vondrasek, J. Annu. Rev. Biophys. Biomol. Struct. 27, 249–284 (1998).
Wlodawer, A. & Erickson, J.W. Annu. Rev. Biochem. 62, 543–585 (1993).
Dunn, B.M., Goodenow, M.M., Gustchina, A. & Wlodawer, A. Genome Biol. 3, S3006.1–S3006.7 (2002).
Gustchina, A., Jaskolski, M. & Wlodawer, A. Cell Cycle 5, 463–464 (2006).
Li, M. et al. Proc. Natl. Acad. Sci. USA 102, 18322–18337 (2005).
Yoshinaka, Y., Katoh, I., Copeland, T.D. & Oroszlan, S. Proc. Natl. Acad. Sci. USA 82, 1618–1622 (1985).
DiMaio, F., Tyka, M.D., Baker, M.L., Chiu, W. & Baker, D. J. Mol. Biol. 392, 181–190 (2009).
Miller, M., Jaskólski, M., Rao, J.K.M., Leis, J. & Wlodawer, A. Nature 337, 576–579 (1989).
Wlodawer, A. et al. Science 245, 616–621 (1989).
Sirkis, R., Gerst, J.E. & Fass, D. J. Mol. Biol. 364, 376–387 (2006).
Tang, J., James, M.N.G., Hsu, I.N., Jenkins, J.A. & Blundell, T.L. Nature 271, 618–621 (1978).
Hartl, M.J., Wohrl, B.M., Rosch, P. & Schweimer, K. J. Mol. Biol. 381, 141–149 (2008).
Louis, J.M., Clore, G.M. & Gronenborn, A.M. Nat. Struct. Biol. 6, 868–875 (1999).
DeLano, W.L. The PyMOL Molecular Graphics System (DeLano Scientific, San Carlos, California, USA, 2002).
Acknowledgements
We acknowledge the use of beamline 22-ID of the Southeast Regional Collaborative Access Team (SER-CAT), located at the Advanced Photon Source (APS), Argonne National Laboratory. Use of the APS was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. W-31-109-Eng-38. This work was supported in part by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research and with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products or organizations imply endorsement by the US Government.
Author information
Authors and Affiliations
Contributions
M.L. produced the protein and grew crystals; M.L. and Z.D. collected and processed crystallographic data; M.L., F.D.M., D.Z., A.G., J.L., and Z.D. performed calculations, structure refinement and analysis; D.B. and A.W. supervised the project. All authors discussed the results and participated in writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1 and 2, and Supplementary Methods (PDF 3481 kb)
Rights and permissions
About this article
Cite this article
Li, M., DiMaio, F., Zhou, D. et al. Crystal structure of XMRV protease differs from the structures of other retropepsins. Nat Struct Mol Biol 18, 227–229 (2011). https://doi.org/10.1038/nsmb.1964
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nsmb.1964
This article is cited by
-
Ddi1-like protein from Leishmania major is an active aspartyl proteinase
Cell Stress and Chaperones (2013)
-
phenix.mr_rosetta: molecular replacement and model rebuilding with Phenix and Rosetta
Journal of Structural and Functional Genomics (2012)