Abstract
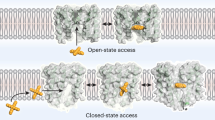
Understanding how ion channels open and close their pores is crucial for comprehending their physiological roles. We used intracellular quaternary ammonium blockers, electrophysiology and X-ray crystallography to locate the voltage-dependent gate in MthK potassium channels from Methanobacterium thermoautotrophicum. Blockers bind in an aqueous cavity between two putative gates: an intracellular gate and the selectivity filter. Thus, these blockers directly probe gate location—an intracellular gate will prevent binding when closed, whereas a selectivity filter gate will always allow binding. Kinetic analysis of tetrabutylammonium block of single MthK channels combined with X-ray crystallographic analysis of the pore with tetrabutyl antimony unequivocally determined that the voltage-dependent gate, like the C-type inactivation gate in eukaryotic channels, is located at the selectivity filter. State-dependent binding kinetics suggest that MthK inactivation leads to conformational changes within the cavity and intracellular pore entrance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hille, B. Ion Channels of Excitable Membranes (Sinauer, Sunderland, Massachusetts, USA, 2001).
Armstrong, C.M. & Binstock, L. Anomalous rectification in the squid giant axon injected with tetraethylammonium chloride. J. Gen. Physiol. 48, 859–872 (1965).
Armstrong, C.M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J. Gen. Physiol. 58, 413–437 (1971).
Choi, K.L., Mossman, C., Aube, J. & Yellen, G. The internal quaternary ammonium receptor site of Shaker potassium channels. Neuron 10, 533–541 (1993).
Liu, Y., Holmgren, M., Jurman, M.E. & Yellen, G. Gated access to the pore of a voltage-dependent K+ channel. Neuron 19, 175–184 (1997).
Holmgren, M., Smith, P.L. & Yellen, G. Trapping of organic blockers by closing of voltage-dependent K+ channels: evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109, 527–535 (1997).
Doyle, D.A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998).
Zhou, M., Morais-Cabral, J.H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001).
Jiang, Y. et al. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522 (2002).
Jiang, Y. et al. The open pore conformation of potassium channels. Nature 417, 523–526 (2002).
Yellen, G. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31, 239–295 (1998).
Hoshi, T., Zagotta, W.N. & Aldrich, R.W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990).
Kurata, H.T. & Fedida, D. A structural interpretation of voltage-gated potassium channel inactivation. Prog. Biophys. Mol. Biol. 92, 185–208 (2006).
Choi, K.L., Aldrich, R.W. & Yellen, G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA 88, 5092–5095 (1991).
McCoy, J.G. & Nimigean, C.M. Structural correlates of selectivity and inactivation in potassium channels. Biochim. Biophys. Acta 1818, 272–285 (2012).
Cuello, L.G., Jogini, V., Cortes, D.M. & Perozo, E. Structural mechanism of C-type inactivation in K+ channels. Nature 466, 203–208 (2010).
Kurata, H.T., Wang, Z. & Fedida, D. NH2-terminal inactivation peptide binding to C-type-inactivated Kv channels. J. Gen. Physiol. 123, 505–520 (2004).
Baukrowitz, T. & Yellen, G. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA 93, 13357–13361 (1996).
Panyi, G. & Deutsch, C. Cross talk between activation and slow inactivation gates of Shaker potassium channels. J. Gen. Physiol. 128, 547–559 (2006).
Panyi, G. & Deutsch, C. Probing the cavity of the slow inactivated conformation of Shaker potassium channels. J. Gen. Physiol. 129, 403–418 (2007).
Flynn, G.E. & Zagotta, W.N. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron 30, 689–698 (2001).
Bruening-Wright, A., Lee, W.S., Adelman, J.P. & Maylie, J. Evidence for a deep pore activation gate in small conductance Ca2+-activated K+ channels. J. Gen. Physiol. 130, 601–610 (2007).
Proks, P., Antcliff, J.F. & Ashcroft, F.M. The ligand-sensitive gate of a potassium channel lies close to the selectivity filter. EMBO Rep. 4, 70–75 (2003).
Contreras, J.E., Srikumar, D. & Holmgren, M. Gating at the selectivity filter in cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA 105, 3310–3314 (2008).
Klein, H. et al. Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis. J. Gen. Physiol. 129, 299–315 (2007).
Wilkens, C.M. & Aldrich, R.W. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128, 347–364 (2006).
Thompson, J. & Begenisich, T. Selectivity filter gating in large-conductance Ca2+-activated K+ channels. J. Gen. Physiol. 139, 235–244 (2012).
Tang, Q.Y., Zeng, X.H. & Lingle, C.J. Closed-channel block of BK potassium channels by bbTBA requires partial activation. J. Gen. Physiol. 134, 409–436 (2009).
Zhou, Y., Xia, X.M. & Lingle, C.J. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl. Acad. Sci. USA 108, 12161–12166 (2011).
Cui, J., Yang, H. & Lee, U.S. Molecular mechanisms of BK channel activation. Cell. Mol. Life Sci. 66, 852–875 (2009).
Yuan, P., Leonetti, M.D., Hsiung, Y. & MacKinnon, R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature 481, 94–97 (2012).
Wu, Y., Yang, Y., Ye, S. & Jiang, Y. Structure of the gating ring from the human large-conductance Ca2+-gated K+ channel. Nature 466, 393–397 (2010).
Zadek, B. & Nimigean, C.M. Calcium-dependent gating of MthK, a prokaryotic potassium channel. J. Gen. Physiol. 127, 673–685 (2006).
Ye, S., Li, Y. & Jiang, Y. Novel insights into K+ selectivity from high-resolution structures of an open K+ channel pore. Nat. Struct. Mol. Biol. 17, 1019–1023 (2010).
Thomson, A.S. & Rothberg, B.S. Voltage-dependent inactivation gating at the selectivity filter of the MthK K+ channel. J. Gen. Physiol. 136, 569–579 (2010).
Martínez-François, J.R. & Lu, Z. Intrinsic versus extrinsic voltage sensitivity of blocker interaction with an ion channel pore. J. Gen. Physiol. 135, 149–167 (2010).
Lenaeus, M.J., Vamvouka, M., Focia, P.J. & Gross, A. Structural basis of TEA blockade in a model potassium channel. Nat. Struct. Mol. Biol. 12, 454–459 (2005).
Yohannan, S., Hu, Y. & Zhou, Y. Crystallographic study of the tetrabutylammonium block to the KcsA K+ channel. J. Mol. Biol. 366, 806–814 (2007).
Faraldo-Gómez, J.D. et al. Mechanism of intracellular block of the KcsA K+ channel by tetrabutylammonium: insights from X-ray crystallography, electrophysiology and replica-exchange molecular dynamics simulations. J. Mol. Biol. 365, 649–662 (2007).
French, R.J. & Shoukimas, J.J. Blockage of squid axon potassium conductance by internal tetra-N-alkylammonium ions of various sizes. Biophys. J. 34, 271–291 (1981).
Guo, D. & Lu, Z. Kinetics of inward-rectifier K+ channel block by quaternary alkylammonium ions. Dimension and properties of the inner pore. J. Gen. Physiol. 117, 395–406 (2001).
Spassova, M. & Lu, Z. Coupled ion movement underlies rectification in an inward-rectifier K+ channel. J. Gen. Physiol. 112, 211–221 (1998).
Pau, V.P., Abarca-Heidemann, K. & Rothberg, B.S. Allosteric mechanism of Ca2+ activation and H+-inhibited gating of the MthK K+ channel. J. Gen. Physiol. 135, 509–526 (2010).
Woodhull, A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61, 687–708 (1973).
Thompson, J. & Begenisich, T. External TEA block of shaker K+ channels is coupled to the movement of K+ ions within the selectivity filter. J. Gen. Physiol. 122, 239–246 (2003).
Heginbotham, L. & Kutluay, E. Revisiting voltage-dependent relief of block in ion channels: a mechanism independent of punchthrough. Biophys. J. 86, 3663–3670 (2004).
Nimigean, C.M. & Miller, C. Na+ block and permeation in a K+ channel of known structure. J. Gen. Physiol. 120, 323–335 (2002).
Rothberg, B.S., Shin, K.S., Phale, P.S. & Yellen, G. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J. Gen. Physiol. 119, 83–91 (2002).
Contreras, J.E. & Holmgren, M. Access of quaternary ammonium blockers to the internal pore of cyclic nucleotide-gated channels: implications for the location of the gate. J. Gen. Physiol. 127, 481–494 (2006).
Contreras, J.E. et al. Voltage profile along the permeation pathway of an open channel. Biophys. J. 99, 2863–2869 (2010).
Jogini, V. & Roux, B. Electrostatics of the intracellular vestibule of K+ channels. J. Mol. Biol. 354, 272–288 (2005).
Geng, Y., Niu, X. & Magleby, K.L. Low resistance, large dimension entrance to the inner cavity of BK channels determined by changing side-chain volume. J. Gen. Physiol. 137, 533–548 (2011).
Chen, X. & Aldrich, R.W. Charge substitution for a deep-pore residue reveals structural dynamics during BK channel gating. J. Gen. Physiol. 138, 137–154 (2011).
Li, W. & Aldrich, R.W. State-dependent block of BK channels by synthesized shaker ball peptides. J. Gen. Physiol. 128, 423–441 (2006).
Wang, D.T., Hill, A.P., Mann, S.A., Tan, P.S. & Vandenberg, J.I. Mapping the sequence of conformational changes underlying selectivity filter gating in the Kv11.1 potassium channel. Nat. Struct. Mol. Biol. 18, 35–41 (2011).
Cuello, L.G. et al. Structural basis for the coupling between activation and inactivation gates in K+ channels. Nature 466, 272–275 (2010).
Adams, P.D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W.G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Winn, M.D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67, 235–242 (2011).
Qin, F., Auerbach, A. & Sachs, F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys. J. 70, 264–280 (1996).
Acknowledgements
The authors thank Y. Jiang for advice on MthK crystal preparation and J. Rubin for technical assistance. B. Rothberg (Temple University, Philadelphia, Pennsylvania) provided the MthK-pQE82 construct. We also thank the staff of the X25 beamline at the National Synchrotron Light Source, Brookhaven National Laboratory, for their help. This work was supported by an NRSA postdoctoral fellowship from the US National Institutes of Health (F32GM087865) to D.J.P. and a National Institutes of Health grant (RO1GM088352) to C.M.N.
Author information
Authors and Affiliations
Contributions
D.J.P. and C.M.N. designed the research; D.J.P. acquired and analyzed single-channel data; D.J.P. and J.G.M. acquired and analyzed crystallographic data; D.J.P., J.G.M. and C.M.N. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplemetary Figures 1–7, Supplementary Tables 1–3 and Supplementary Note (PDF 1695 kb)
Rights and permissions
About this article
Cite this article
Posson, D., McCoy, J. & Nimigean, C. The voltage-dependent gate in MthK potassium channels is located at the selectivity filter. Nat Struct Mol Biol 20, 159–166 (2013). https://doi.org/10.1038/nsmb.2473
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/nsmb.2473
This article is cited by
-
An LQT2-related mutation in the voltage-sensing domain is involved in switching the gating polarity of hERG
BMC Biology (2024)
-
Calcium-gated potassium channel blockade via membrane-facing fenestrations
Nature Chemical Biology (2024)
-
Inferring functional units in ion channel pores via relative entropy
European Biophysics Journal (2021)
-
A constricted opening in Kir channels does not impede potassium conduction
Nature Communications (2020)
-
Lipid-protein interactions modulate the conformational equilibrium of a potassium channel
Nature Communications (2020)