Abstract
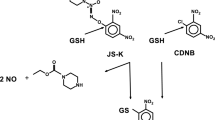
Nitric oxide (NO) is a major effector molecule in cancer prevention. A number of studies have shown that NO prodrug JS-K (O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate) induces apoptotic cell death in vitro and in vivo, indicating that it is a promising new therapeutic for cancer. However, the mechanism of its tumor-killing activity remains unclear. Ubiquitin plays an important role in the regulation of tumorigenesis and cell apoptosis. Our earlier report has shown that inactivation of the ubiquitin system through blocking E1 (ubiquitin-activating enzyme) activity preferentially induces apoptosis in p53-expressing transformed cells. As E1 has an active cysteine residue that could potentially interact with NO, we hypothesized that JS-K could inactivate E1 activity. E1 activity was evaluated by detecting ubiquitin-E1 conjugates through immunoblotting. JS-K strikingly inhibits the ubiquitin-E1 thioester formation in cells in a dose-dependent manner with an IC50 of approximately 2 μM, whereas a JS-K analog that cannot release NO did not affect these levels in cells. Moreover, JS-K decreases total ubiquitylated proteins and increases p53 levels, which is mainly regulated by ubiquitin and proteasomal degradation. Furthermore, JS-K preferentially induces cell apoptosis in p53-expressing transformed cells. These findings indicate that JS-K inhibits E1 activity and kills transformed cells harboring wild-type p53.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Abbreviations
- E1:
-
ubiquitin-activating enzyme
- NO:
-
nitric oxide
- RPE:
-
Tert-immortalized human retinal pigment epithelial cells
References
Adams J . (2004). The development of proteasome inhibitors as anticancer drugs. Cancer Cell 5: 417–421.
Bandara LR, La Thangue NB . (1991). Adenovirus E1a prevents the retinoblastoma gene product from complexing with a cellular transcription factor. Nature 351: 494–497.
Chen J, Lin J, Levine AJ . (1995). Regulation of transcription functions of the p53 tumor suppressor by the mdm-2 oncogene. Mol Med 1: 142–152.
Chen ZJ . (2005). Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol 7: 758–765.
Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M . (2006). S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science 314: 467–471.
Giarre M, Semenov MV, Brown AM . (1998). Wnt signaling stabilizes the dual-function protein beta-catenin in diverse cell types. Ann N Y Acad Sci 857: 43–55.
Hershko A, Ciechanover A . (1998). The ubiquitin system. Annu Rev Biochem 67: 425–479.
Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH . (2001). Protein S-nitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol 3: 193–197.
Jahngen-Hodge J, Obin MS, Gong X, Shang F, Nowell Jr TR, Gong J et al. (1997). Regulation of ubiquitin-conjugating enzymes by glutathione following oxidative stress. J Biol Chem 272: 28218–28226.
Jansen AP, Camalier CE, Colburn NH . (2005). Epidermal expression of the translation inhibitor programmed cell death 4 suppresses tumorigenesis. Cancer Res 65: 6034–6041.
Kimelman D, Xu W . (2006). Beta-catenin destruction complex: insights and questions from a structural perspective. Oncogene 25: 7482–7491.
Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L et al. (2007). JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood 110: 709–718.
Lipton SA . (1999). Neuronal protection and destruction by NO. Cell Death Differ 6: 943–951.
Liu J, Li C, Qu W, Leslie E, Bonifant CL, Buzard GS et al. (2004). Nitric oxide prodrugs and metallochemotherapeutics: JS-K and CB-3-100 enhance arsenic and cisplatin cytolethality by increasing cellular accumulation. Mol Cancer Ther 3: 709–714.
Lowe SW, Ruley HE, Jacks T, Housman DE . (1993b). p53-Dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell 74: 957–967.
Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T . (1993a). p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362: 847–849.
Ren Z, Kar S, Wang Z, Wang M, Saavedra JE, Carr BI . (2003). JS-K, a novel non-ionic diazeniumdiolate derivative, inhibits Hep 3B hepatoma cell growth and induces c-Jun phosphorylation via multiple MAP kinase pathways. J Cell Physiol 197: 426–434.
Saavedra JE, Srinivasan A, Buzard GS, Davies KM, Waterhouse DJ, Inami K et al. (2006). PABA/NO as an anticancer lead: analogue synthesis, structure revision, solution chemistry, reactivity toward glutathione, and in vitro activity. J Med Chem 49: 1157–1164.
Shami PJ, Saavedra JE, Bonifant CL, Chu J, Udupi V, Malaviya S et al. (2006). Antitumor activity of JS-K [O2-(2,4-dinitrophenyl) 1-[(4-ethoxycarbonyl)piperazin-1-yl]diazen-1-ium-1,2-diolate] and related O2-aryl diazeniumdiolates in vitro and in vivo. J Med Chem 49: 4356–4366.
Shami PJ, Saavedra JE, Wang LY, Bonifant CL, Diwan BA, Singh SV et al. (2003). JS-K, a glutathione/glutathione S-transferase-activated nitric oxide donor of the diazeniumdiolate class with potent antineoplastic activity. Mol Cancer Ther 2: 409–417.
Simeone AM, McMurtry V, Nieves-Alicea R, Saavedra JE, Keefer LK, Johnson MM et al. (2008). TIMP-2 mediates the anti-invasive effects of the nitric oxide-releasing prodrug JS-K in breast cancer cells. Breast Cancer Res 10: R44.
Turchi JJ . (2006). Nitric oxide and cisplatin resistance: NO easy answers. Proc Natl Acad Sci USA 103: 4337–4338.
Yang Y, Kitagaki J, Dai RM, Tsai YC, Lorick KL, Ludwig RL et al. (2007). Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res 67: 9472–9481.
Yang Y, Li CC, Weissman AM . (2004). Regulating the p53 system through ubiquitination. Oncogene 23: 2096–2106.
Yang Y, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV et al. (2005). Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell 7: 547–559.
Ying L, Hofseth LJ . (2007). An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res 67: 1407–1410.
Acknowledgements
We are grateful to the NIH Fellows Editorial Board for revisions of this paper. This work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and by National Cancer Institute Contract No. NOI-CO-12400 to SAIC Inc. JK was a fellow of the Japanese Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kitagaki, J., Yang, Y., Saavedra, J. et al. Nitric oxide prodrug JS-K inhibits ubiquitin E1 and kills tumor cells retaining wild-type p53. Oncogene 28, 619–624 (2009). https://doi.org/10.1038/onc.2008.401
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2008.401
Keywords
This article is cited by
-
Structural and Functional Characterisation of the Domains of Ubiquitin-Activating Enzyme (E1) of Saccharomyces cerevisiae
Cell Biochemistry and Biophysics (2020)
-
JS-K, a nitric oxide donor, induces autophagy as a complementary mechanism inhibiting ovarian cancer
BMC Cancer (2019)
-
Targeting the neddylation pathway in cells as a potential therapeutic approach for diseases
Cancer Chemotherapy and Pharmacology (2018)
-
JS-K, a nitric oxide pro-drug, regulates growth and apoptosis through the ubiquitin-proteasome pathway in prostate cancer cells
BMC Cancer (2017)
-
Nitric oxide released from JS-K induces cell death by mitotic catastrophe as part of necrosis in glioblastoma multiforme
Cell Death & Disease (2016)