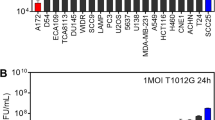
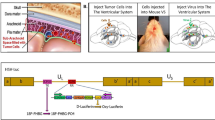
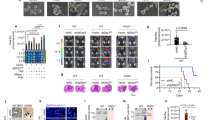
Abstract
Oncolytic herpes simplex viruses (HSVs), in clinical trials for the treatment of malignant gliomas, are assumed to be selective for tumor cells because their replication is strongly attenuated in quiescent cells, but not in cycling cells. Oncolytic selectivity is thought to occur because mutations in viral ICP6 (encoding a viral ribonucleotide reductase function) and/or γ34.5 function are respectively complemented by mammalian ribonucleotide reductase and GADD34, whose genes are expressed in cycling cells. However, it is estimated that only 5–15% of malignant glioma cells are in mitosis at any one time. Therefore, effective replication of HSV oncolytic viruses might be limited to a subpopulation of tumor cells, since at any one time the majority of tumor cells would not be cycling. However, we report that an HSV with defective ICP6 function replicates in quiescent cultured murine embryonic fibroblasts obtained from mice with homozygous p16 deletions. Furthermore, intracranial inoculation of this virus into the brains of p16−/− mice provides evidence of viral replication that does not occur when the virus is injected into the brains of wild-type mice. These approaches provide in vitro and in vivo evidence that ICP6-negative HSVs are ‘molecularly targeted,’ because they replicate in quiescent tumor cells carrying specific oncogene deletions, independent of cell cycle status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aghi M, Martuza RL . (2005). Oncolytic viral therapies—the clinical experience. Oncogene 24: 7802–7816.
Burns KL, Ueki K, Jhung SL, Koh J, Louis DN . (1998). Molecular genetic correlates of p16, cdk4, and pRb immunohistochemistry in glioblastomas. J Neuropathol Exp Neurol 57: 122–130.
Costello JF, Berger MS, Huang HS, Cavenee WK . (1996). Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 56: 2405–2410.
Elledge SJ, Zhou Z, Allen JB . (1992). Ribonucleotide reductase: regulation, regulation, regulation. Trends Biochem Sci 17: 119–123.
Farassati F, Yang AD, Lee PW . (2001). Oncogenes in Ras signalling pathway dictate host-cell permissiveness to herpes simplex virus 1. Nat Cell Biol 3: 745–750.
Finney DJ. . (1971). Probit Analysis. Cambridge University Press: New York, NY.
Fu X, Tao L, Cai R, Prigge J, Zhang X . (2006). A mutant type 2 herpes simplex virus deleted for the protein kinase domain of the ICP10 gene is a potent oncolytic virus. Mol Ther 13: 882–890.
Goldstein DJ, Weller SK . (1988). Factor(s) present in herpes simplex virus type 1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology 166: 41–51.
Gray-Bablin J, Rao S, Keyomarsi K . (1997). Lovastatin induction of cyclin-dependent kinase inhibitors in human breast cells occurs in a cell cycle-independent fashion. Cancer Res 57: 604–609.
Hoshino T, Prados M, Wilson CB, Cho KG, Lee KS, Davis RL . (1989). Prognostic implications of the bromodeoxyuridine labeling index of human gliomas. J Neurosurg 71: 335–341.
Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D et al. (1999). Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med 5: 881–887.
Jiang H, Gomez-Manzano C, Alemany R, Medrano D, Alonso M, Bekele BN et al. (2005). Comparative effect of oncolytic adenoviruses with E1A-55 kDa or E1B-55 kDa deletions in malignant gliomas. Neoplasia 7: 48–56.
Kamiryo T, Tada K, Shiraishi S, Shinojima N, Nakamura H, Kochi M et al. (2002). Analysis of homozygous deletion of the p16 gene and correlation with survival in patients with glioblastoma multiforme. J Neurosurg 96: 815–822.
Kirn D, Martuza RL, Zwiebel J . (2001). Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med 7: 781–787.
Komata T, Kondo Y, Koga S, Ko SC, Chung LW, Kondo S . (2000). Combination therapy of malignant glioma cells with 2-5A-antisense telomerase RNA and recombinant adenovirus p53. Gene Therapy 7: 2071–2079.
McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK et al. (2001). Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res 61: 8751–8757.
Nakamura M, Konishi N, Hiasa Y, Tsunoda S, Nakase H, Tsuzuki T et al. (1998). Frequent alterations of cell-cycle regulators in astrocytic tumors as detected by molecular genetic and immunohistochemical analyses. Brain Tumor Pathol 15: 83–88.
Nakamura T, Peng KW, Harvey M, Greiner S, Lorimer IA, James CD et al. (2005). Rescue and propagation of fully retargeted oncolytic measles viruses. Nat Biotechnol 23: 209–214.
Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW . (2004). Reovirus oncolysis: the Ras/RalGEF/p38 pathway dictates host cell permissiveness to reovirus infection. Proc Natl Acad Sci USA 101: 11099–11104.
Ohgaki H, Kleihues P . (2007). Genetic pathways to primary and secondary glioblastoma. Am J Pathol 170: 1445–1453.
Ono Y, Tamiya T, Ichikawa T, Kunishio K, Matsumoto K, Furuta T et al. (1996). Malignant astrocytomas with homozygous CDKN2/p16 gene deletions have higher Ki-67 proliferation indices. J Neuropathol Exp Neurol 55: 1026–1031.
O'Shea CC, Soria C, Bagus B, McCormick F . (2005). Heat shock phenocopies E1B-55 K late functions and selectively sensitizes refractory tumor cells to ONYX-015 oncolytic viral therapy. Cancer Cell 8: 61–74.
Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ et al. (2001). Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature 413: 86–91.
Smith KD, Mezhir JJ, Bickenbach K, Veerapong J, Charron J, Posner MC et al. (2006). Activated MEK suppresses activation of PKR and enables efficient replication and in vivo oncolysis by Deltagamma(1)34.5 mutants of herpes simplex virus 1. J Virol 80: 1110–1120.
Stojdl DF, Lichty B, Knowles S, Marius R, Atkins H, Sonenberg N et al. (2000). Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med 6: 821–825.
Ueki K, Ono Y, Henson JW, Efird JT, Von Deimling A, Louis DN . (1996). CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res 56: 150–153.
Wang CC, Liao YP, Mischel PS, Iwamoto KS, Cacalano NA, McBride WH . (2006). HDJ-2 as a target for radiosensitization of glioblastoma multiforme cells by the farnesyltransferase inhibitor R115777 and the role of the p53/p21 pathway. Cancer Res 66: 6756–6762.
Watanabe T, Nakamura M, Yonekawa Y, Kleihues P, Ohgaki H . (2001). Promoter hypermethylation and homozygous deletion of the p14ARF and p16INK4a genes in oligodendrogliomas. Acta Neuropathol (Berl) 101: 185–189.
Wein LM, Wu JT, Kirn DH . (2003). Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: implications for virus design and delivery. Cancer Res 63: 1317–1324.
Wurdinger T, Verheije MH, Raaben M, Bosch BJ, De Haan CA, Van Beusechem VW et al. (2005). Targeting non-human coronaviruses to human cancer cells using a bispecific single-chain antibody. Gene Therapy 12: 1394–1404.
Yoon SS, Carroll NM, Chiocca EA, Tanabe KK . (1999). Influence of p53 on herpes simplex virus type 1 vectors for cancer gene therapy. J Gastrointest Surg 3: 34–48.
Acknowledgements
This study was funded by an American Brain Tumor Association Fellowship to MA and by NIH PO1 CA69246, R01 NS41571, R01 CA85139 to EAC. RAD was supported by an ACS Research Professor Award and NIH U01 CA084628 and P01 CA095616.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghi, M., Visted, T., DePinho, R. et al. Oncolytic herpes virus with defective ICP6 specifically replicates in quiescent cells with homozygous genetic mutations in p16. Oncogene 27, 4249–4254 (2008). https://doi.org/10.1038/onc.2008.53
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2008.53
Keywords
This article is cited by
-
Clinical trial links oncolytic immunoactivation to survival in glioblastoma
Nature (2023)
-
CRISPR/Cas9 and next generation sequencing in the personalized treatment of Cancer
Molecular Cancer (2022)
-
Antitumor effects of IL-12 and GM-CSF co-expressed in an engineered oncolytic HSV-1
Gene Therapy (2021)
-
Biological basis for novel mesothelioma therapies
British Journal of Cancer (2021)
-
Designing herpes viruses as oncolytics
Molecular Therapy - Oncolytics (2015)