Abstract
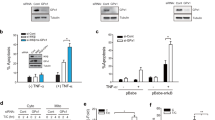
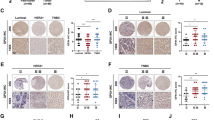
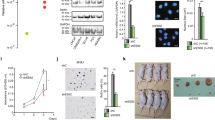
The complexity of gene regulation has created obstacles to defining mechanisms that establish the patterns of gene expression characteristic of the different clinical phenotypes of breast cancer. TFAP2C is a transcription factor that has a critical role in the regulation of both estrogen receptor-alpha (ERα) and c-ErbB2/HER2 (Her2). Herein, we performed chromatin immunoprecipitation and direct sequencing (ChIP-seq) for TFAP2C in four breast cancer cell lines. Comparing the genomic binding sites for TFAP2C, we identified that glutathione peroxidase (GPX1) is regulated by TFAP2C through an AP-2 regulatory region in the promoter of the GPX1 gene. Knockdown of TFAP2C, but not the related factor TFAP2A, resulted in an abrogation of GPX1 expression. Selenium-dependent GPX activity correlated with endogenous GPX1 expression and overexpression of exogenous GPX1 induced GPX activity and significantly increased resistance to tert-butyl hydroperoxide. Methylation of the CpG island encompassing the AP-2 regulatory region was identified in cell lines where TFAP2C failed to bind the GPX1 promoter and GPX1 expression was unresponsive to TFAP2C. Furthermore, in cell lines where GPX1 promoter methylation was associated with gene silencing, treatment with 5′-aza-2-deoxycytidine (5′-aza-dC) (an inhibitor of DNA methylation) allowed TFAP2C to bind to the GPX1 promoter resulting in the activation of GPX1 RNA and protein expression. Methylation of the GPX1 promoter was identified in ∼20% of primary breast cancers and a highly significant correlation between the TFAP2C and GPX1 expression was confirmed when considering only those tumors with an unmethylated promoter, whereas the related factor, TFAP2A, failed to demonstrate a correlation. The results demonstrate that TFAP2C regulates the expression of GPX1, which influences the redox state and sensitivity to oxidative stress induced by peroxides. Given the established role of GPX1 in breast cancer, the results provide an important mechanism for TFAP2C to further influence oncogenesis and progression of breast carcinoma cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Jemal A, Siegel R, Xu J, Ward E . Cancer statistics, 2010. CA Cancer J Clin 2010; 60: 277–300.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001; 98: 10869–10874.
Buzdar AU . Role of biologic therapy and chemotherapy in hormone receptor- and HER2-positive breast cancer. Ann Oncol 2009; 20: 993–999.
Williams T, Admon A, Luscher B, Tjian R . Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev 1988; 2: 1557–1569.
Bosher JM, Totty NF, Hsuan JJ, Williams T, Hurst HC . A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene 1996; 13: 1701–1707.
Feng W, Williams T . Cloning and characterization of the mouse AP-2 epsilon gene: a novel family member expressed in the developing olfactory bulb. Mol Cell Neurosci 2003; 24: 460–475.
Moser M, Imhof A, Pscherer A, Bauer R, Amselgruber W, Sinowatz F et al. Cloning and characterization of a second AP-2 transcription factor: AP-2 beta. Development 1995; 121: 2779–2788.
Zhao F, Satoda M, Licht JD, Hayashizaki Y, Gelb BD . Cloning and characterization of a novel mouse AP-2 transcription factor, AP-2delta, with unique DNA binding and transactivation properties. J Biol Chem 2001; 276: 40755–40760.
Eckert D, Buhl S, Weber S, Jager R, Schorle H . The AP-2 family of transcription factors. Genome Biol 2005; 6: 246.
McPherson LA, Baichwal VR, Weigel RJ . Identification of ERF-1 as a member of the AP2 transcription factor family. Proc Natl Acad Sci USA 1997; 94: 4342–4347.
Woodfield GW, Horan AD, Chen Y, Weigel RJ . TFAP2C controls hormone response in breast cancer cells through multiple pathways of estrogen signaling. Cancer Res 2007; 67: 8439–8443.
Tan SK, Lin ZH, Chang CW, Varang V, Chng KR, Pan YF et al. AP-2gamma regulates oestrogen receptor-mediated long-range chromatin interaction and gene transcription. EMBO J 2011; 30: 2569–2581.
Begon DY, Delacroix L, Vernimmen D, Jackers P, Winkler R . Yin Yang 1 cooperates with activator protein 2 to stimulate ERBB2 gene expression in mammary cancer cells. J Biol Chem 2005; 280: 24428–24434.
Delacroix L, Begon D, Chatel G, Jackers P, Winkler R . Distal ERBB2 promoter fragment displays specific transcriptional and nuclear binding activities in ERBB2 overexpressing breast cancer cells. DNA Cell Biol 2005; 24: 582–594.
Yang JW, Lee EY, Kang KW . ErbB2 overexpression in p53-inactivated mammary epithelial cells. FEBS Lett 2006; 580: 6501–6508.
Ailan H, Xiangwen X, Daolong R, Lu G, Xiaofeng D, Xi Q et al. Identification of target genes of transcription factor activator protein 2 gamma in breast cancer cells. BMC Cancer 2009; 9: 279.
Allouche A, Nolens G, Tancredi A, Delacroix L, Mardaga J, Fridman V et al. The combined immunodetection of AP-2alpha and YY1 transcription factors is associated with ERBB2 gene overexpression in primary breast tumors. Breast Cancer Res 2008; 10: R9.
Turner BC, Zhang J, Gumbs AA, Maher MG, Kaplan L, Carter D et al. Expression of AP-2 transcription factors in human breast cancer correlates with the regulation of multiple growth factor signalling pathways. Cancer Res 1998; 58: 5466–5472.
Pellikainen J, Naukkarinen A, Ropponen K, Rummukainen J, Kataja V, Kellokoski J et al. Expression of HER2 and its association with AP-2 in breast cancer. Eur J Cancer 2004; 40: 1485–1495.
Woodfield GW, Chen Y, Bair TB, Domann FE, Weigel RJ . Identification of primary gene targets of TFAP2C in hormone responsive breast carcinoma cells. Genes Chromosomes Cancer 2010; 49: 948–962.
Woodfield GW, Hitchler MJ, Chen Y, Domann FE, Weigel RJ . Interaction of TFAP2C with the estrogen receptor-alpha promoter is controlled by chromatin structure. Clin Cancer Res 2009; 15: 3672–3679.
Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J et al. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 2009; 4: e6146.
Jee CD, Kim MA, Jung EJ, Kim J, Kim WH . Identification of genes epigenetically silenced by CpG methylation in human gastric carcinoma. Eur J Cancer 2009; 45: 1282–1293.
Brown NS, Bicknell R . Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res 2001; 3: 323–327.
Liou GY, Storz P . Reactive oxygen species in cancer. Free Radic Res 2010; 44: 479–496.
Schumacker PT . Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 2006; 10: 175–176.
Maiti AK . Genetic determinants of oxidative stress-mediated sensitization of drug-resistant cancer cells. Int J Cancer 2012; 130: 1–9.
Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X et al. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature 2011; 475: 231–234.
Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC . Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med 2000; 28: 754–766.
Hu YJ, Diamond AM . Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res 2003; 63: 3347–3351.
Ravn-Haren G, Olsen A, Tjonneland A, Dragsted LO, Nexo BA, Wallin H et al. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis 2006; 27: 820–825.
Cox DG, Tamimi RM, Hunter DJ . Gene x Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer 2006; 6: 217.
Zhuo P, Goldberg M, Herman L, Lee BS, Wang H, Brown RL et al. Molecular consequences of genetic variations in the glutathione peroxidase 1 selenoenzyme. Cancer Res 2009; 69: 8183–8190.
Hu J, Zhou GW, Wang N, Wang YJ . GPX1 Pro198Leu polymorphism and breast cancer risk: a meta-analysis. Breast Cancer Res Treatment 2010; 124: 425–431.
Hu Y, Benya RV, Carroll RE, Diamond AM . Allelic loss of the gene for the GPX1 selenium-containing protein is a common event in cancer. J Nutr 2005; 135: 3021S–3024SS.
Townsend AJ, Morrow CS, Sinha BK, Cowan KH . Selenium-dependent glutathione peroxidase expression is inversely related to estrogen receptor content of human breast cancer cells. Cancer Commun 1991; 3: 265–270.
Jenssen TK, Kuo WP, Stokke T, Hovig E . Associations between gene expressions in breast cancer and patient survival. Hum Genet 2002; 111: 411–420.
Gouaze V, Mirault ME, Carpentier S, Salvayre R, Levade T, Andrieu-Abadie N . Glutathione peroxidase-1 overexpression prevents ceramide production and partially inhibits apoptosis in doxorubicin-treated human breast carcinoma cells. Mol Pharmacol 2001; 60: 488–496.
Vibet S, Goupille C, Bougnoux P, Steghens JP, Gore J, Maheo K . Sensitization by docosahexaenoic acid (DHA) of breast cancer cells to anthracyclines through loss of glutathione peroxidase (GPx1) response. Free Radic Biol Med 2008; 44: 1483–1491.
Gao J, Xiong Y, Ho YS, Liu X, Chua CC, Xu X et al. Glutathione peroxidase 1-deficient mice are more susceptible to doxorubicin-induced cardiotoxicity. Biochim Biophys Acta 2008; 1783: 2020–2029.
Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell 2006; 10: 241–252.
Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R . Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011; 20: 300–314.
Thewes V, Orso F, Jager R, Eckert D, Schafer S, Kirfel G et al. Interference with activator protein-2 transcription factors leads to induction of apoptosis and an increase in chemo- and radiation-sensitivity in breast cancer cells. BMC Cancer 2010; 10: 192.
Yu L, Hitchler MJ, Sun W, Sarsour EH, Goswami PC, Domann FE . TFAP2A inhibits c-MYC induced oxidative stress and apoptosis in HaCaT human keratinocytes. J Oncol 2009; 2009: 780874.
Zhu CH, Huang Y, Oberley LW, Domann FE . A family of AP-2 proteins down-regulate manganese superoxide dismutase expression. J Biol Chem 2001; 276: 14407–14413.
Hussain SP, Amstad P, He P, Robles A, Lupold S, Kaneko I et al. p53-induced up-regulation of MnSOD and GPx but not catalase increases oxidative stress and apoptosis. Cancer Res 2004; 64: 2350–2356.
McPherson LA, Loktev AV, Weigel RJ . Tumor Suppressor Activity of AP2alpha Mediated through a Direct Interaction with p53. J Biol Chem 2002; 277: 45028–45033.
Scibetta AG, Wong PP, Chan KV, Canosa M, Hurst HC . Dual association by TFAP2A during activation of the p21cip/CDKN1A promoter. Cell Cycle 2010; 9: 4525–4532.
Hannay JA, Liu J, Zhu QS, Bolshakov SV, Li L, Pisters PW et al. Rad51 overexpression contributes to chemoresistance in human soft tissue sarcoma cells: a role for p53/activator protein 2 transcriptional regulation. Mol Cancer Ther 2007; 6: 1650–1660.
Lacroix M, Leclercq G . Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat 2004; 83: 249–289.
Edgar R, Domrachev M, Lash AE . Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 2002; 30: 207–210.
McPherson LA, Weigel RJ . AP2alpha and AP2gamma: a comparison of binding site specificity and transactivation of the estrogen receptor promoter and single site promoter constructs. Nucleic Acids Res 1999; 27: 4040–4049.
Johansson K, Jarvliden J, Gogvadze V, Morgenstern R . Multiple roles of microsomal glutathione transferase 1 in cellular protection: a mechanistic study. Free Radic Biol Med 2010; 49: 1638–1645.
Acknowledgements
We appreciate tumor samples provided by Dr Ryan Askeland and Christine Hochstedler (Department of Pathology, University of Iowa) and statistical support from Dr Junlin Liao (Department of Surgery, University of Iowa). This work was supported in part by the National Institutes of Health grants R01CA109294 (PI: RJ Weigel) and R01CA115438 (PI: FE Domann). Anthony Cyr received salary support from NIH F30 AA019856 and Dr Philip Spanheimer was supported by the NIH grant T32CA148062 (PI: RJ Weigel). The work was also supported by a generous gift from the Kristen Olewine Milke Breast Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kulak, M., Cyr, A., Woodfield, G. et al. Transcriptional regulation of the GPX1 gene by TFAP2C and aberrant CpG methylation in human breast cancer. Oncogene 32, 4043–4051 (2013). https://doi.org/10.1038/onc.2012.400
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2012.400
Keywords
This article is cited by
-
Crucial role of the transcription factors family activator protein 2 in cancer: current clue and views
Journal of Translational Medicine (2023)
-
TFAP2C regulates carbonic anhydrase XII in human breast cancer
Oncogene (2020)
-
Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3β/Snail signaling
Oncogene (2018)
-
Identification of differentially expressed genes regulated by molecular signature in breast cancer-associated fibroblasts by bioinformatics analysis
Archives of Gynecology and Obstetrics (2018)
-
The role of Tcfap2c in tumorigenesis and cancer growth in an activated Neu model of mammary carcinogenesis
Oncogene (2015)