Abstract
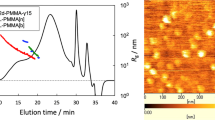
New chiral methacrylates, (S)-methylbenzyl methacryloyloxyethyl urea (MBMOU) and (S)-methoxycarbonylbenzylmethyl methacryloyloxyethyl urea (MCMOU) were synthesized from 2-(methacryloyloxy)ethyl isocyanate (MOI) and (S)-methylbenzylamine and l-phenylalanine methyl ester, respectively. Radical polymerizations of MBMOU and MCMOU were performed under several conditions to obtain the corresponding polymers whose specific optical rotations ([α]25435) were -13.5° to -10.9° and 30.8° to 31.5°, respectively. From the results of radical copolymerizations of RMOU (MBMOU and MCMOU, M1) with styrene (ST, M2) or butyl methacrylate (BMA, M2), monomer reactivity ratios (r1, r2) and Alfrey–Price Q-e were determined. The chiroptical properties of poly(MBMOU-co-M2)s were strongly influenced by co-units. Poly(RMOU)-bonded-silica gel as chiral stationary phase (CSP) was prepared for high performance liquid chromatography (HPLC). The CSPs resolved some racemates such as ketoprofen and ethyl mandelate. The enantiorecognition ability may be based on higher-ordered structures of the polymer.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
S. Matsuhiro, Konbatekku, 5, 8 (1997).
H. Kamogawa, H. Kohno, and R. Kitagawa, J. Polym. Sci., Part A: Polym. Chem., 27, 487 (1989).
C. Decker and K. Moussa, Eur. Polym. J., 27, 881 (1991).
I. Francis, S. Arjen, C. Israel, and S. Johannes, J. Polym. Sci., Part A: Polym. Chem., 31, 239 (1993).
H. Albrecht and W. Wunderlich, Angew. Makromol. Chem., 145/146, 89 (1986).
S. Savaskan and B. Hazer, Angew. Makromol. Chem., 239, 13 (1996).
N. Monzner, U. K. Fischer, T. Volkel, and V. Rheinberger, Angew. Makromol. Chem., 245, 155 (1997).
T. Oishi, Y.-K. Lee, A. Nakagawa, K. Onimura, and H. Tsutsumi, Polym. J., 33, 81 (2001).
C. H. Bamford, K. G. Al-Lamee, I. P. Middleton, J. Paprotny, and R. Carr, Bull. Soc. Chim. Belg., 99, 919 (1990).
Y. Kimitsuka, K. Iwata, H. Suzuki, Jpn. Kokai Tokkyo Koho, JP 6-308108 (Nov. 4, 1994).
Y. K. Lee, K. Onimura, H. Tsutsumi, and T. Oishi, J. Polym. Sci., Part A: Polym. Chem., 38, 4315 (2000).
Y. K. Lee, K. Onimura, H. Tsutsumi, and T. Oishi, Polym. J., 32, 1007 (2000).
Y. K. Lee, K. Onimura, H. Tsutsumi, and T. Oishi, Polym. J., 33, 411 (2001).
S. G. Allenmark, “Chromatographic Enantioseparation”, Ellis Horwood Limited., Chichester, UK, 1988.
For example, Y. Okamoto, CHEMTECH, 176 (1987).
J. H. Liu and F. J. Tsai, J. Appl. Polym. Sci., 72, 677 (1999).
Y. Tamai, P. Qian, K. Matsunaga, and S. Miyano, Bull. Chem. Soc. Jpn., 65, 817 (1992).
G. Blaschke, Angew. Chem. Int. Ed. Eng., 19, 13 (1980).
G. Blaschke, W. Broker, and E. Frankel, Angew. Chem. Int. Ed. Eng., 25, 830 (1986).
D. Arlt, B. Bomer, R. Grosser, and W. Lange, Angew. Chem. Int. Ed. Eng., 30, 1662 (1991).
T. Otsu and M. Kinoshita, “Koubunshigousei Jikkenhou”, Kagaku-doujin Publishing Co., Ltd., Kyoto, 1972, pp 69–91.
N. Izumiya and T. Kato, “Pepuchidogousei no Kiso to Jikken”, Maruzen, Tokyo, 1985, pp 50–51.
Y. Okamoto, E. Yashima, M. Ishihara, and K. Hatada, Bull. Chem. Soc. Jpn., 61, 255 (1988).
F. Tüdös, T. Kelen, T. Foldes-Berezsnich, and B. Turcsanyi, J. Macromol. Sci. Chem., A10, 1513 (1976). The monomer reactivity rations were determined by the method 6 described in this paper.
T. Alfrey, Jr., and C. C. Price, J. Polym. Sci., 2, 101 (1947).
H. J. Harwood and W. M. Ritchey, J. Polym. Sci., Polym. Lett. Ed., 2, 601 (1964).
M. Kurokawa, H. Yamaguchi, and Y. Minoura, J. Polym. Sci., Chem. Ed., 17, 2241 (1979).
R. N. Majumdar, C. Carlini, and C. Bertucci, Makromol. Chem., 183, 2047 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, YK., Hisamitsu, N., Onimura, K. et al. Synthesis of Novel Chiral Poly(methacrylate)s Having Urea Moieties and (S)-Methylbenzyl or l-Phenylalanine Methyl Ester Groups and Their Chiral Recognition Abilities. Polym J 34, 9–17 (2002). https://doi.org/10.1295/polymj.34.9
Issue date:
DOI: https://doi.org/10.1295/polymj.34.9