Abstract
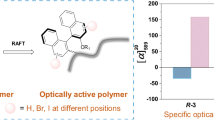
N-Phenylmaleimide (PhMI) and its derivatives were polymerized using chiral anionic initiators consisting of diethylzinc (Et2Zn) and chiral ligands to investigate the effect of the bulkiness, flexibility and polarity of the ortho- or para-substituents on optical activity of the obtained polymer. The optical activity of the polymer was extremely influenced by N-substituents, initiators, and other polymerization conditions. Poly(N-2-biphenylmaleimide) (poly(2-BPMI)) formed with Et2Zn-(–)-2,2’-(1-ethylpropylidene)bis(4-benzyl-2-oxazoline) (Bnbox) showed the highest specific rotation ([α]25435) of +164.7 °. From 13C NMR spectroscopy, optically active poly(2-BPMI) and poly(N-(2-phenoxy)-phenylmaleimide) (poly(2-POPMI)) mainly possessed threo-diisotactic structures in the main chains. Chiral stationary phase prepared from optically active poly(PhMI) optically resolved racemic 1,1’-bi-2-naphthol.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
T. Oishi, H. Yamasaki, and M. Fujimoto, Polym. J., 23, 795 (1991).
K. Onimura, H. Tsutsumi, and T. Oishi, Polym. Bull., 39, 437 (1997).
K. Onimura, H. Tsutsumi, and T. Oishi, Macromolecules, 31, 5971 (1998).
K. Onimura, H. Tsutsumi, and T. Oishi, Chem. Lett., 791 (1998).
T. Oishi, K. Onimura, K. Tanaka, W. Horimoto, and H. Tsutsumi, J. Polym. Sci., Part A: Polym. Chem., 37, 473 (1999).
H. Zhou, K. Onimura, H. Tsutsumi, and T. Oishi, Polym. J., 32, 552 (2000).
Y. Isobe, K. Onimura, T. Oishi, and H. Tsutsumi, Polym. J., 32, 1052 (2000).
Y. Okamoto, T. Nakano, H. Kobayashi, and K. Hatada, Polym. Bull., 25, 5 (1991).
W. Liu, C. Chen, Y. Chen, and F. Xi, J. Macromol. Sci., Pure Appl. Chem., 34, 327 (1997).
W. Liu, C. Chen, Y. Chen, and F. Xi, Polym. Bull., 38, 509 (1997).
T. Oishi, K. Onimura, Y. Isobe, H. Yanagihara, and H. Tsutsumi, J. Polym. Sci., Part A: Polym. Chem., 38, 310 (2000).
T. Oishi, K. Onimura, Y. Isobe, and H. Tsutsumi, Chem. Lett., 673 (1999).
Y. Isobe, K. Onimura, H. Tsutsumi, and T. Oishi, J. Polym. Sci. Part A: Polym. Chem., 39, 3556 (2001).
Y. Isobe, K. Onimura, H. Tsutsumi, and T. Oishi, Macromolecule, 34, 7617 (2001).
R. C. P. Cubbon, Polymer, 6, 419 (1965).
P. Y. Reddy, S. Kondo, T. Toru, and Y. Ueno, J. Org. Chem., 62, 2652 (1997).
P. Y. Reddy, S. Kondo, S. Fujita, and T. Toru, Synthesis, 999 (1998).
M. Strukelj and A. S. Hay, Macromolecules, 25, 4721 (1992).
M. S. Chisholm, J. G. Carey, M. E. B. Jones, and P. Wade, Polymer, 33, 838 (1992).
M. S. Chisholm, J. G. Carey, M. E. B. Jones, and P. Wade, Polymer, 33, 842 (1992).
A. Abiko and S. Masamune, Tetrahedron Lett., 33, 5517 (1992).
S. E. Denmark, N. Nakajima, O. J.-C. Nicaise, A.-M. Faucher, and J. P. Edwards, J. Org. Chem., 60, 4884 (1995).
Y. Isobe, K. Onimura, H. Tsutsumi, and T. Oishi, Polym. J., 32, 1052 (2000).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Oishi, T., Isobe, Y., Onimura, K. et al. Asymmetric Polymerization of N-ortho- or para-Substituted Phenylmaleimide Using Chiral Anionic Initiators. Polym J 35, 245–254 (2003). https://doi.org/10.1295/polymj.35.245
Issue date:
DOI: https://doi.org/10.1295/polymj.35.245
Keywords
This article is cited by
-
Direct observation of cyclic poly(N-substituted maleimide)s with broad size distributions synthesized by anionic polymerization using an N-heterocyclic carbene and successive ring closure without high dilutions
Polymer Journal (2020)
-
Synthesis and properties of N-substituted maleimides conjugated with 1,4-phenylene or 2,5-thienylene polymers
Polymer Journal (2010)