Abstract
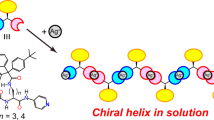
The novel styrene derivatives bearing various amino groups at ortho-position, 2-(1-indolinyl)methylstyrene (2), 2-(9-carbazolyl)methylstyrene (3), 2-(N,N-diphenylamino)methylstyrene (4), 2-(N-3-methoxyphenyl-N-phenylamino)methylstyrene (5), and 2-(N-3-methylphenyl-N-phenylamino)methylstyrene (6), were synthesized and polymerized with radical and anionic initiations. As the bulkiness of the substituents at the ortho-position increased, the polymerizabilities of these monomers during the radical and anionic methods significantly decreased. The anionic polymerization of 4–6 with the n-butyllithium (−)-sparteine complex in toluene at 0 °C afforded the optically active polymers ([α]25365 +48° to +22°), whereas no optical activity was observed for the polymers of 2 and 3 obtained under the same conditions. The optically active polymers likely have a chiral helical conformation which is related to a high stereoregularity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
For example: M. Yokoyama, J. Synth. Org. Chem. Jpn., 28, 443 (1970).
Y. Suto, Kogyo Kagaku Zasshi, 67, 380 (1964).
F. Millich and C. E. Carraher Jr., J. Polym. Sci., Part A-1: Polym. Chem., 7, 2669 (1969).
F. Millich and C. E. Carraher Jr., J. Polym. Sci., Part A-1: Polym. Chem., 8, 163 (1970).
F. Millich and C. E. Carraher Jr., Macromollecules, 3, 253 (1970).
F. Millich and L. L. Lambing, J. Polym. Sci., Polym. Chem. Ed., 18, 2155 (1980).
Y. Imai, N. Sato, and M. Ueda, Makromol. Chem., Rapid Commun., 1, 419 (1980).
Y. Imai, H. Kamata, and M. Kakimoto, J. Polym. Sci., Polym. Chem. Ed., 22, 1259 (1984).
M. Richard, B. I. Dahiyat, D. M. Arm, S. Lim, and K. W. Leong, J. Polym. Sci., Part A: Polym. Chem., 29, 1157 (1991).
T. Nishikubo, A. Kameyama, and S. Minegishi, Macromolecules, 27, 2641 (1994).
T. Nishikubo, A. Kameyama, and S. Minegishi, Macromolecules, 28, 4810 (1995).
T. Nishikubo, A. Kameyama, and N. Hayashi, Polym. J., 25, 1003 (1993).
T. Nishikubo, A. Kameyama, Y. Kimura, and K. Fukuyo, Macromolecules, 28, 4361 (1995).
S. Minegishi, A. Kameyama, and T. Nishikubo, React. Funct. Polym., 30, 317 (1996).
RadTech Japan, Ed., “Ultraviolet and Electron Beam Curable Materials,” CMC, Tokyo, 1992.
J. V. Crivello, J. L. Lee, and D. A. Colon, Radiation Curing VI: Conference Proceeding; Association for Finishing Processes: Chicago, IL, 1982, pp 4–28.
T. Nishikubo, Y. Hayashi, T. Iizawa, T. Sasaki, T. Mastumoto, and T. Fukudome, J. Appl. Polym. Sci., 44, 107 (1992).
J. V. Crivello and K. D. Jo, J. Polym. Sci., Part A: Polym. Chem., 31, 1473 (1993).
J. V. Crivello and K. D. Jo, J. Polym. Sci., Part A: Polym. Chem., 31, 1483 (1993).
J. V. Crivello and W.-G. Kim, J. Polym. Sci., Part A: Polym. Chem., 32, 1639 (1994).
J. V. Crivello and G. Lohden, J. Polym. Sci., Part A: Polym. Chem., 34, 2051 (1996).
H. Itoh, A. Kameyama, and T. Nishikubo, Macromolecules, 28, 883 (1995).
H. Itoh, A. Kameyama, and T. Nishikubo, J. Polym. Sci., Part A: Polym. Chem., 34, 217 (1996).
H. Itoh, A. Kameyama, and T. Nishikubo, J. Polym. Sci., Part A: Polym. Chem., 35, 3217 (1997).
W. R. Watt, H. T. Hoffman!!Jr!! M. Pobinar, L. J. Scholnick, and L. S. Yang, J. Polym. Sci., Polym. Chem. Ed., 22, 1789 (1984).
T. Nishikubo and A. Kameyama, Prog. Polym. Sci., 18, 963 (1993).
K. Meier and H. Zweifel, J. Radiat. Curing, 26, (4), 13 (1986).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ajiro, H., Habaue, S. & Okamoto, Y. Anionic Polymerization of Novel Styrene Derivatives Bearing Various Amino Groups at ortho-Position. Polym J 36, 323–328 (2004). https://doi.org/10.1295/polymj.36.323
Published:
Issue date:
DOI: https://doi.org/10.1295/polymj.36.323