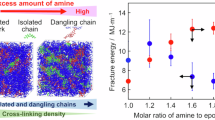
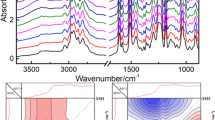
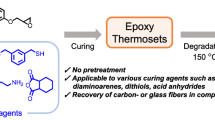
Abstract
Imidazole derivatives and alkylamines are very useful as hardeners of epoxy resins. These amine compounds form inclusion complexes with 1,1,2,2-tetrakis(4-hydroxyphenyl) ethane (TEP). When these amine-containing inclusion complexes are reacted with epoxy resins, curing acceleration, pot life extension, and higher curing temperatures are observed.An explanation for this curing behavior is that guest molecule “amine compounds” are trapped in inclusion complex crystals. X-Ray crystal structure data shows the existence of O-H–N hydrogen bonding between host and guest molecules. With the imidazole molecule in the inclusion complex, hydrogen bonding prevents easy attack of the epoxy ring. Amine-TEP inclusion complexes show these enhanced curing behaviors when reacted with epoxy resins.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
H. Suzuki, Tetrahedron Lett., 35, 5015 (1994).
H. Suzuki and H. Takagi, Tetrahedron Lett., 34, 4805 (1993).
H. Suzuki, H. Takagi, and R. Sato, Tetrahedron Lett. 38, 4563 (1997).
K. Tanaka and F. Toda, Chem. Rev., 100, 1025 (2000).
J. L. Atwood, J. E. D. Davies, F. Vogtle, J.-M. Lean, D. D. Macnicol, F. Toda, and R. Bishop, “Comprehensive Supramolecular Chemistry”, Volume 6, PERGAMON, 1996.
M. Yagi, S. Hirano, S. Toyota, F. Toda, P. Giastas, and I. M. Marvidis, Heterocycles, 59, 735 (2003).
S. Nagahama, K. Inoue, K. Sada, M. Mikiya, and A. Matsumoto, Cryst. Growth Des., 3, 247 (2003).
H. Kaku, S. Tanaka, and T. Tsunoda, Tetrahedron, 58, 3401 (2002).
K. Kato, M. Sugahara, N. Tohnai, K. Sada, and M. Mikiya, Eur. J. Org. Chem., 981 (2004).
Y. Takai, T. Nakaoki, T. Takaeda, and M. Sawada, Tetrahedron, 58, 4319 (2002).
M. Farina, G. Allegra, and G. Natta, J. Am. Chem. Soc., 86, 516 (1964).
K. Kishikawa, S. Tsubokura, S. Kohmoto, and M. Yamamoto, J. Org. Chem., 64, 7568 (1999).
T. Hens, Q. Li, and T. C. W. Mak, Eur. J. Org. Chem., 1115 (1999).
M. R. Caira, A. Jacobs, L. R. Nassimbeni, and F. Toda, Cryst. Eng. Comm., 150 (2003).
F. Guo, W. S. Guo, and F. Toda, Cryst. Eng. Comm., 5, 45 (2003).
S. Hirano, K. Yoshizawa, S. Toyota, F. Toda, and Z. Urabancyk-Lipkowska, Mendeleev Commun., 141 (2003).
K. Tanaka, M. Asami, and J. L. Scott, New J. Chem., 26, 378 (2002).
F. Toda, M. Senzaki, and R. Kuroda, Chem. Commun., 1788 (2002).
D. E. Lynch, G. Smith, K. A. Byriel, and C. H. Kennard, J. Chem. Soc., Chem. Commun., 300 (1992).
N. Amanokura, T. Sahara, and R. Sato, Anal. Sci., 11, x273 (2006).
N. Amanokura, M. Kaneko, T. Sahara, and R. Sato, Anal. Sci., in press.
The inclusion complex was expressed as a host·guest.
The 1a·2k single crystal structure was prepared slowly from methanol.
Refer to the experimental section.
The case using 1a·2k and 1a·2l as catalysts indicated the most remarkable difference between only the imidazoles and the inclusion complexes in each experiment.
G. J. Buist, J. M. Barton, B. J. Howlin, J. R. Jones, and M. J. Parker, J. Mater. Chem., 6, 911 (1996).
V. Spacek, J. Pouchly, and J. Biros, Eur. Polym. J., 23, 377 (1987).
T. Kagurai and T. Noguchi, Kougyou kagaku Zasshi, 63, 98 (1960).
L. Shechter, J. Wynstra, and R. P. Kurkjy, Ind. Eng. Chem., 48, 94 (1956).
Refer to the experimental section.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amanokura, N., Kaneko, M., Sahara, T. et al. Curing Behavior of Epoxy Resin Initiated by Amine-Containing Inclusion Complexes. Polym J 39, 845–852 (2007). https://doi.org/10.1295/polymj.PJ2006224
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1295/polymj.PJ2006224