Abstract
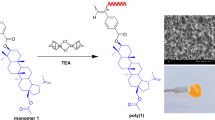
Seven novel types of acetylene monomers, 4-oxo-4-(prop-2-ynyloxy)butanoic acid ester derivatives (PSRs), having ester groups of 4′-substituted phenyl (PSPRs: –H=PSP; –CN=PSPCN; –OCH3=PSPOC1) or 4′-substituted biphenyl (PSBPRs: –H=PSBP; –CN=PSBPCN; –OCH3=PSBPOC1; –O(CH2)5CH3=PSBPOC6) as a pendant group were synthesized from 4-oxo-4-(prop-2-ynyloxy)butanoic acid (PS) with phenol derivatives and polymerized with a rhodium-catalyzed system. The structures and properties of the polymers were characterized and evaluated by nuclear magnetic resonance, infrared spectroscopy and gel permeation chromatography analyses. The polymerizations of the PSRs were carried out under nitrogen with Rh catalysts in tetrahydrofuran or toluene at 30 °C, producing a good yield of polymers. The resulting polymer showed a thermotropic mesophase as observed through polarized optical microscopy and differential scanning calorimetry.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Ho, M.- S. & Hsu, C.- S. Synthesis and hierarchical superstructures of side-chain liquid crystal polyacetylenes containing galactopyranoside end-groups. J. Polym. Sci. Part A Polym. Chem. 47, 6596–6611 (2009).
Hsu, C.- S. The application of side-chain liquid-crystalline polymers. Prog. Polym. Sci. 22, 829–871 (1997).
Yashima, E., Matsushima, T. & Okamoto, Y. Poly((4-carboxyphenyl)acetylene) as a probe for chirality assignment of amines by circular dichroism. J. Am. Chem. Soc. 117, 11596–11597 (1995).
Yashima, E. & Maeda, K. Chirality-responsive helical polymers. Macromolecules 41, 3–12 (2008).
Kishimoto, Y., Itou, M., Miyatake, T., Ikariya, T. & Noyori, R. Polymerization of monosubstituted acetylenes with a zwitterionic rhodium(I) complex, Rh+(2,5-norbornene)[(η6-C6H5)B−(C6H5)3]. Macromolecules 28, 6662–6666 (1995).
Koltzenburg, S., Wolff, D., Stelzer, F., Springer, J. & Nuyken, O. Investigation of the thermal and morphological behavior of liquid-crystalline acetylene homo- and copolymers. Macromolecules 31, 9166–9173 (1998).
Kusabayashi, S. in EKISHOU ZAIRYO 67–94 (Kodansha, Tokyo, 1991).
Goto, T., Shiba, T. & Matsuura, T. in YUUKIJIKKEN NO TEBIKI 1, (Kagakudoujin, Kyoto, 1988).
Schrock, R. R. & Osborn, J. A. π-Bonded complexes of the tetraphenylborate ion with rhodium(I) and iridium(I). Inorg. Chem. 9, 2339–2343 (1970).
Altintas, O., Hizal, G. & Tunca, U. ABC-type hetero-arm star terpolymers through ‘click’ chemistry. J. Polym. Sci. Part A Polym. Chem. 44, 5699–5707 (2006).
Abdul Karim, S. M., Nomura, R. & Masuda, T. Degradation behavior of stereoregular Cis-transoidal poly(phenylacetylene)s. J. Polym. Sci. Part A Polym. Chem. 39, 3130–3136 (2001).
Akagi, K., Piao, G., Kaneko, S., Sakamaki, K., Shirakawa, H. & Kyotani, M. Helical polyacetylene synthesized with a chiral nematic reaction field. Science 282, 1683–1686 (1998).
Goto, H., Nimori, S. & Akagi, K. Synthesis and properties of mono-substituted liquid crystalline polyacetylene derivatives-doping, magnetic orientation, and photo-isomerization. Synth. Met. 155, 576–587 (2005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mizuta, K., Hiraga, N., Koga, T. et al. Synthesis of side-chain liquid crystalline polyacetylenes bearing succinic acid spacer. Polym J 43, 141–146 (2011). https://doi.org/10.1038/pj.2010.120
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2010.120
Keywords
This article is cited by
-
Synthesis of chiral side-chain liquid crystalline polyacetylenes bearing succinic acid spacer
Polymer Bulletin (2012)