Abstract
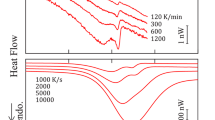
The melting behavior and crystallization kinetics of PBN–PDEN and PBN–PTDEN copolymers were investigated using differential scanning calorimetry. Multiple endotherms were observed in all of the copolymers under investigation, originating from melting and recrystallization processes. By applying the Hoffman–Weeks method, the Tm° of the α and β′-PBN phases were derived. The Tm° value of the β′-form, which has not been determined before, is significantly higher, as expected, because the β′-phase is thermodynamically favored and more tightly packed. The isothermal crystallization kinetics were analyzed according to the Avrami treatment. The presence of either oxygen or sulfur atoms in the PBN polymeric chain was found to reduce its crystallizability. In particular, the crystallization rate regularly decreased as the co-unit content was increased. Lastly, the α-PBN phase was found to crystallize faster than β′-one, which is expected, as it the more kinetically favored phase.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Watanabe, H. Stretching and structure of polybutylene-naphthalene-2, 6-dicarboxylate films. Kobunshi. Ronbunshu. 33, 229–237 (1976).
Ju, M. Y., Huang, J. M. & Chang, F. C. Crystal polymorphism of poly(butylene-2,6-naphthalate) prepared by thermal treatments. Polymer 43, 2065–2074 (2002).
Soccio, M., Gazzano, M., Lotti, N., Finelli, L. & Munari, A. Copolymerization: a new tool to selectively induce poly(butylene naphthalate) crystal form. Polymer 51, 192–200 (2010).
Soccio, M., Gazzano, M., Lotti, N., Finelli, L. & Munari, A. Synthesis and characterization of novel random copolymers based on PBN: influence of thiodiethylene naphthalate co-units on its polymorphic behaviour. Polymer 51, 192–200 (2010).
Righetti, M. C. & Munari, A. Influence of branching on melting behavior and isothermal crystallization of poly(butylene terephthalate). Macromol. Chem. Phys. 198, 363–378 (1997).
Marand, H., Alizadeh, A., Farmer, R., Desai, R. & Velikov, V. Influence of structural and topological constraints on the crystallization and melting behavior of polymers. 2. Poly(arylene ether ether ketone). Macromolecules 33, 3392–3403 (2000).
Chung, J. S. & Cebe, P. Melting behavior of poly(phenylene sulfide). 2. Multiple-stage melt crystallization. Polymer 33, 2312–2324 (1992).
Lemstra, P. J., Schouten, A. J. & Challa, G. Secondary crystallization of isotatic polystyrene. J. Polym. Sci. Polym. Phys. Ed. 12, 1565–1574 (1974).
Minakov, A. A., Mordvinsted, D. A. & Schick, C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer 45, 3755–3763 (2004).
Kong, Y. & Hay, J. N. Multiple melting behaviour of poly(ethylene terephthalate). Polymer 44, 623–633 (2002).
Yasuniwa, M., Tsubakihara, S., Fujioka, T. & Dan, Y. X-ray studies of multiple melting behavior of poly(butylene-2,6-naphthalate). Polymer 46, 8306–8312 (2005).
Papageorgiou, G. & Karayannidis, G. Multiple melting behaviour of poly(ethylene-co-butylene naphthalate-2,6-dicarboxylate)s. Polymer 40, 5325–5332 1999.
Ju, M. & Chang, F. C. Multiple melting behavior of poly(butylene-2,6-naphthalate). Polymer 42, 5037–5045 (2001).
Yasuniwa, M., Tsubakihara, S. & Fujioka, T. X-ray and DSC studies on the melt-recrystallization process of poly(butylenes naphthalate). Thermochim. Acta. 396, 75–78 (2003).
Hoffman, J. D. & Weeks, J. J. Melting process and equilibrium melting temperature of poly(chlorotrifluoroethylene). J. Res. Natl. Bur. Stand. 66A, 13–28 (1962).
Marand, H., Xu, J. & Srinivas, S. Determination of the equilibrium melting temperature of polymer crystals: linear and nonlinear hoffman-weeks extrapolations. Macromolecules 31, 8219–8229 (1998).
Xu, J., Srinivas, S., Marand, H. & Agarwal, P. Equilibrium melting temperature and undercooling dependence of the spherulitic growth rate of isotactic polypropylene. Macromolecules 31, 8230–8242 (1998).
Wu, P. L. & Woo, E. M. Linear versus nonlinear determinations of equilibrium melting temperatures of poly(trimethylene terephthalate) and miscible blend with poly(ether imide) exhibiting multiple melting peaks. J. Polym. Sci. Part B Polym. Phys. 40, 1571–1581 (2002).
Al-Hussein, M. & Strobl, G. The melting line, the crystallization line, and the equilibrium melting temperature of isotactic polystyrene. Macromolecules 35, 1672–1676 (2002).
Finelli, L., Lotti, N., Munari, A., Gazzano, M. & Malta, V. Poly(thiodiethylene adipate): melting behavior, crystallization kinetics, morphology, and crystal structure. J. Polym. Sci. Part B Polym. Phys. 42, 553–566 (2004).
Fichera, A. M., Finelli, L., Gazzano, M., Lotti, N. & Munari, A. Multiple melting behaviour of poly(thiodiethylene terephthalate): further investigations by means of X-ray and thermal techniques. Macromol. Chem. Phys. 205, 63–72 (2004).
Flory, P. J. Theory of crystallization in copolymers. Trans. Faraday Soc. 51, 848–857 (1955).
Baur, H. & Baltorowicz, M. Influence of sequence-length. Distribution on the melting end point of copolymers. Makromol. Chem. 98, 297–301 (1966).
Sanchez, I. C. & Eby, R. K. Thermodynamics and crystallization of random copolymers. Macromolecules 8, 638–641 (1975).
Helfand, E. & Lauritzen, J. I. Theory of copolymer crystallization. Macromolecules 6, 631–638 (1973).
Gazzano, M., Soccio, M., Lotti, N., Finelli, L. & Munari, A. J. Therm. Anal. Cal. (submitted for publication).
Avrami, M. J. Granulation, phase change and microstructure. Kinetics of phase change III. J. Chem. Phys. Chem. Phys. 9, 177–184 (1941).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soccio, M., Lotti, N., Finelli, L. et al. Equilibrium melting temperature and crystallization kinetics of α- and β′-PBN crystal forms. Polym J 44, 174–180 (2012). https://doi.org/10.1038/pj.2011.112
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2011.112
Keywords
This article is cited by
-
Melt Crystallization of Poly(butylene 2,6-naphthalate)
Chinese Journal of Polymer Science (2020)