Abstract
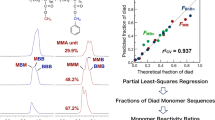
Condensations of polyhydroxyl monomers with terephthalaldehyde or isophthalaldehyde give the corresponding polyspiroacetals. The effects of various dialdehydes and multihydroxy monomers on the properties of the resulting polymer have been examined. Model compounds were synthesized by the condensation of multihydroxy monomers with benzaldehyde. The model compounds and polymers were characterized by Fourier transform infrared spectroscopy, nuclear magnetic resonance (NMR), thermogravimetric analysis and differential scanning calorimetry. The proposed polymer structure is supported by the solid-state CPMAS 13C NMR spectrum of the model compound. The synthesized polyspiroacetals are thermally stable, have a high degree of chemical stability and are soluble in hexafluoroisopropanol.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bailey, W. J. Encyclopedia of Polymer Science and Engineering 158 (Wiley, New York, 1993).
Nakamura, S. Polymeric Materials Encylopedia Vol. 10 (ed. Salamone J.C.) 7854 (Academic press, New York, 1996).
Sonmez, H. B. & Wudl, F. Thermally stable spiro polymers. Polym. Prep. 44, 803 (2003).
Bailey, W. J. & Volpe, A. A. Synthesis of spiro polymers. J. Polym. Sci. 8, 2109–2122 (1970).
Bailey, W. J., Beam, C. F., Cappuccilli, Jr E.D., Haddad, I. & Volpe, A. A. Synthesis of polyspiroketals containing five-, six-, seven- and eight- membered rings. ACS Sympossium Ser. 195, 391–402 (1982).
Cohen, S. M. & Lavin, E. Polyspiroacetals resins. Part I. Initial preparation and characterization. J. Appl. Polym. Sci. 6, 503–507 (1962).
Cohen, S. M., Hunt, C. F., Kass, R. E. & Markhart, A. H. Polyspiroacetal resins. Part II. Structure and properties of polyspiroacetals from pentaerythritol-glutaraldehyde and from (pentaerythritol-dipentaerythritol)-glutaraldehyde. J. Appl. Polym. Sci. 6, 508–517 (1962).
Lee, K., Kim, H. M. & Rhee, J. M. Synthesis and properties of processable rigid polymers containing spiroacetals moieties. Macromol. Chem. 192, 1033–1040 (1991).
Maslinska-Solich, J. & Kukowa, S. Synthesis of poly(spiroacetal-ether)s. Polym. Int. 52, 1633–1640 (2003).
Makhseed, S. & McKeown, N. B. Novel spiro-polymers with enhanced solubility. Chem. Comm. 3, 255–256 (1999).
Mannich, C. & Brose, W. Über die synthese von ketoalkoholen und mehrwertigen alkoholen aus cyclischen ketonen und formaldehyd. Chem. Ber. 56, 833 (1923).
Witcoff, H. Tetramethylolcyclohexanol. Inorg. Synth. Coll. 4, 907 (1963)Vol. 31, p.101 (1951).
Ray, G. C. US Patent 2.500.570. Chem. Abst. 34, 5385 (1950).
Feuerbacher, N., Vogtle, F., Windscheidt, J., Poetsch, E. & Nieger, M. Synthesis of rodlike dispiro hydrocarbon skeletons for new liquid crystal compounds. Synthesis 1, 117–120 (1999).
Beam, C. F. & Bailey, W. J. A convenient preparation of cyclohexane-1,4-dicarbaldehyde and cyclo-hexane-1,1,4,4-tetramethanol. J. Chem. Soc., C: Organic. 2730 (1971).
Acknowledgements
We thank the Gebze Institute of Technology Research Foundation for financial support (Project No: 2007-A-03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sonmez, H., Kuloglu, F., Karadag, K. et al. Terephthalaldehyde- and isophthalaldehyde-based polyspiroacetals. Polym J 44, 217–223 (2012). https://doi.org/10.1038/pj.2011.126
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2011.126
Keywords
This article is cited by
-
Synthesis, characterization and properties of novel polyspiroacetals
Journal of Polymer Research (2013)