Abstract
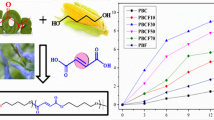
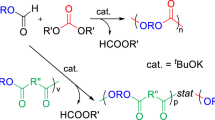
Isopropyl and tert-butyl propargyl carbonates (1b and 1c) were investigated as monomers for Pd(0)-catalyzed polycondensation with bisphenols. These carbonates afforded high-molecular-weight polyethers compared with methyl propargyl carbonate (1a). The number-average molecular weight (Mn) values of the obtained polyethers increased in the order of methyl carbonate 1a<isopropyl carbonate 1b<tert-butyl carbonate 1c. Pd(0)-catalyzed condensation reaction of 1a–c with p-methoxyphenol was carried out as a model reaction. The yield of the desired model product increased with increasing bulkiness of the ester group of 1 (1a<1b<1c). tert-Butyl propargyl carbonate 1c was the most effective for Pd(0)-catalyzed polycondensation with bisphenols. The use of slightly excess 1c yielded higher-molecular-weight polyethers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tsuji, J. Palladium Reagents and Catalysts, New Perspectives for the 21st Century 543–563 (John Wiley: Chichester, UK, 2004).
Tsuji, J. & Mandai, T. Palladium-catalyzed reactions of propargylic compounds in organic synthesis. Angew. Chem. Int. Ed. Engl. 34, 2589–2612 (1995).
Tsuji, J., Watanabe, H., Minami, I. & Shimizu, I. Novel palladium-catalyzed reactions of propargyl carbonates with carbonucleophiles under neutral conditions. J. Am. Chem. Soc. 107, 2196–2198 (1985).
Minami, I., Yuhara, M., Watanabe, H. & Tsuji, J. A new furan annelation by the palladium-catalyzed reaction of 2-alkynyl carbonates or 2-(1-alkynyl)oxiranes with β-keto esters. J. Organomet. Chem. 334, 225–242 (1987).
Labrosse, J. R., Lhoste, P. & Sinou, D. Palladium-catalyzed synthesis of 2,3-dihydro-2-ylidene-1,4-benzodioxins. J. Org. Chem. 66, 6634–6642 (2001).
Dominczak, N., Damez, C., Rhers, B., Labrosse, J. R., Lhoste, P., Kryczka, B. & Sinou, D. Asymmetric palladium-catalyzed annulation of benzene-1,2-diol and racemic secondary propargylic carbonates bearing two different substituents. Tetrahedron 61, 2589–2599 (2005).
Dominczak, N., Lhoste, P., Kryczka, B. & Sinou, D. Palladium-catalyzed heteroannulation of catechol with functionalized propargylic carbonates: influence of the functional group on the regioselectivity of the cyclization. J. Mol. Cat. A Chem. 264, 110–118 (2007).
Uemura, K., Kawaguchi, T., Takayama, H., Nakamura, A. & Inoue, Y. Preparation of alkylidene cyclic carbonates via cyclization of propargylic carbonates. J. Mol. Cat. A Chem. 139, 1–9 (1999).
Yoshida, M., Fujita, M., Ishii, T. & Ihara, M. A novel methodology for the synthesis of cyclic carbonates based on the palladium-catalyzed cascade reaction of 4-methoxycarbonyloxy-2-butyn-1-ols with phenols, involving a novel carbon dioxide elimination-fixation process. J. Am. Chem. Soc. 125, 4874–4881 (2003).
Yoshida, M., Morishita, Y., Fujita, M. & Ihara, M. Palladium-catalyzed cyclization reactions of propargylic carbonates with nucleophiles: a methodology for the syntheses of substituted 2,3-dihydrofurans and benzofurans. Tetrahedron 61, 4381–4393 (2005).
Yoshida, M., Higuchi, M. & Shishido, K. Highly diastereoselective synthesis of tetrahydrobenzofuranones by palladium-catalyzed reaction of propargylic carbonates with 2-substituted cyclohexane-1,3-diones. Tetrahedron Lett. 49, 1678–1681 (2008).
Kozawa, Y. & Mori, M. Novel synthesis of carbapenam by intramolecular attack of lactams nitrogen toward η1-allenyl and η3-propargylpalladium complex. J. Org. Chem. 68, 8068–8074 (2003).
Ren, Z. H., Guan, Z. H. & Liang, Y. M. Highly regioselective synthesis of benz[a]anthracene derivatives via a Pd-catalyzed tandem C-H activation/biscyclization reaction. J. Org. Chem. 74, 3145–3147 (2009).
Koizumi, T., Sugie, K., Kiyonaga, O., Yamanaka, M. & Kawabata, S. Novel palladium – catalyzed polycondensation of propargyl carbonates and bisphenols: synthesis of polyethers having exomethylene groups. Macromolecules 37, 9670–9672 (2004).
Takemura, T., Sugie, K., Nishino, H., Kawabata, S. & Koizumi, T. Pd(0)-catalyzed polycondensation of methyl propargyl carbonate and bisphenols under stoichiometrically imbalanced conditions. J. Polym. Sci., Part A: Polym. Chem. 46, 2250–2261 (2008).
Haight, A. R., Stoner, E. J., Peterson, M. J. & Grover, V. K. General method for the palladium-catalyzed allylation of aliphatic alcohols. J. Org. Chem. 68, 8092–8096 (2003).
Stoner, E. J., Peterson, M. J., Allen, M. S., DeMattei, J. A., Haight, A. R., Leanna, M.R., Patel, S. R., Plata, D. J., Premchandran, R. H. & Rasmussen, M. Allylation of erythromycin derivatives: introduction of allyl substituents into highly hindered alcohols. J. Org. Chem. 68, 8847–8852 (2003).
Wu, M. J., Wei, L. M., Lin, C. F., Leou, S. P. & Wei, L. L. Palladium-catalyzed reactions of aryl iodides with trimethylsilylacetylenes and disubstituted alkynes: the synthesis of diarylacetylenes and triarylethylenes. Tetrahedron 57, 7839–7844 (2001).
Zask, A. & Helquist, P. Palladium hydrides in organic synthesis: reduction of aryl halides by sodium methoxide catalyzed by tetrakis(triphenylphosphine)palladium. J. Org. Chem. 43, 1619–1620 (1978).
Nomura, N., Tsurugi, K. & Okada, M. Mechanistic rationale of a palladium-catalyzed allylic substitution polymerization: carbon-carbon bond-forming polycondensation out of stoichiometric control by cascade bidirectional allylation. Angew. Chem. Int. Ed. 40, 1932–1935 (2001).
Tsurugi, K., Nomura, N. & Aoi, K. Palladium-catalyzed allylic substitution reaction: oxidative addition versus dissociation in an olefin-palladium(0)-complex. Tetrahedron Lett. 43, 469–472 (2002).
Nomura, N., Tsurugi, K., Yoshida, N. & Okada, M. Palladium-catalyzed allylic substitution reaction in polymer synthesis. Curr. Org. Synth. 2, 21–38 (2005).
Kihara, N., Komatsu, S., Takata, T. & Endo, T. Significance of stoichiometric imbalance in step polymerization via reactive intermediate. Macromolecules 32, 4776–4783 (1999).
Iimori, H., Shibasaki, Y., Ando, S. & Ueda, M. Nonstoichiometric polycondensation I. synthesis of polythioether from dibromomethane and 4,4’-thiobisbenzenethiol. Macromol. Symp. 199, 23–26 (2003).
Zhao, D. & Moore, J. S. Nucleationelongation polymerization under imbalanced stoichiometry. J. Am. Chem. Soc. 125, 16294–9 (2003).
Su, C. C., Chen, J. T., Lee, G. H. & Wang, Y. Direct approach to palladium-mediated cycloaddition. First single-crystal structure and convenient synthesis of zwitterionic η3-trimethylenemethane-palladium from nucleophilic addition of carbanions to an Allenyl complex. J. Am. Chem. Soc. 116, 4999–5000 (1994).
Casey, C. P., Nash, J. R., Yi, C. S., Selmeczy, A. D., Chung, S., Powell, D. R. & Hayashi, R. K. Kinetic addition of nucleophiles to η3-propargyl rhenium complexes occurs at the central carbon to produce rhenacylcyclobutenes. J. Am. Chem. Soc. 120, 722–733 (1998).
Tsutsumi, K., Ogoshi, S., Nishiguchi, S. & Kurosawa, H. Synthesis, structure, and reactivity of neutral η3-propargylpalladium complexes. J. Am. Chem. Soc. 120, 1938–1939 (1998).
Nishioka, N. & Koizumi, T. Selective synthesis of functionalized allylic compounds by Pd(0)-catalyzed three-component reaction of methyl propargyl carbonate with phenols and nucleophiles. Tetrahedron Lett. 52, 3662–3665 (2011).
Organ, M. G., Miller, M. & Konstantinou, Z. Mechanism of nucleophilic attack on 1- and 2-bromo(π-allyl)palladium complexes. J. Am. Chem. Soc. 120, 9283–9290 (1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Nishino, H., Nishioka, N. & Koizumi, T. Polycondensation behavior between propargyl carbonates having a bulky ester group and bisphenols in the presence of Pd(0) catalyst: synthesis of exomethylene-containing polyethers. Polym J 44, 321–326 (2012). https://doi.org/10.1038/pj.2011.135
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2011.135