Abstract
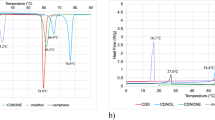
Cardanol novolac (CDN) was synthesized by the reaction of cardanol (CD) and paraformaldehyde in the presence of oxalic acid. The prepolymerized compounds of CD/4,4′-bismaleimidediphenylmethane (BMI) and CDN/BMI with CD/maleimide unit ratios 1/2, 1/4 and 1/6 at 200 °C were finally compression-molded at 250 °C for 5 h to produce cured CD/BMI (cCD/BMI) and cured CDN/BMI (cCDN/BMI) resins. Although the proton nuclear magnetic resonance (1H-NMR) and Fourier transform infrared spectroscopy (FTIR) analyses of the model reaction product of CD and N-phenylmaleimide (PMI) at 200 °C for 8 h suggested the occurrence of the ene reaction and subsequent Diels–Alder reaction, the FTIR analysis of cCD/BMI and cCDN/BMI suggested the occurrence of the ene reaction and addition copolymerization. The cCD/BMI and cCDN/BMI with CD/maleimide ratio lower than 1/2 did not show glass transition until 300 °C and had a 5% weight loss temperature higher than 450 °C. The cCD/BMI and cCDN/BMI with CD/maleimide ratio 1/4 showed the most balanced flexural properties (flexural strength 60–80 MPa, flexural modulus 2.0–2.5 GPa).
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sun, X. S. in Bio-Based Polymers and Composites eds Wool R. P., Sun X. S., Ch. 1, 1–14 (Elsevier, New York, 2005).
Kaplan, D. L in Biopolymers from Renewable Resources ed. Kaplan D. L., Ch.1, 1-29 (Springer, Berlin (1998).
Yu, L., Petinakis, S., Dean, K., Bilyk, A. & Wu, D. Green polymeric blends and composites from renewable resources. Macromol. Symp. 249/250, 535–539 (2007).
Yu, L., Dean, K. & Li, L. Polymer blends and composites from renewable resources. Prog. Polym. Sci. 31, 576–602 (2006).
Ronda, J. C., Lligadas, G., Galià, M. & Cádiz, V. A renewable approach to thermosetting resins. React. Funct. Polym. (advance online publication 24/2012).
Kim, J. R. & Sharma, S. The development and comparison of bio-thermoset plastics from epoxidized plant oils. Ind. Crop. Prod. 36, 485–499 (2012).
Raquez, J. M., Deléglise, M., Lacrampe, M. F. & Krawczak, P. Thermosetting (Bio)materials derived from renewable resources: a critical review. Prog. Polym. Sci. 35, 487–509 (2010).
Effendi, A., Gerhauser, H. & Bridgwater, A. V. Production of renewable phenolic resins by thermochemical conversion of biomass: a review. Renew. Sustain. Energy Rev. 12, 2092–2116 (2008).
Wool, R. P. in Bio-Based Polymers and Composites (eds. Wool R. P., Sun X. S., Ch 7, 202–255 (Elsevier, New York (2005).
Nair, C. P. R. Advances in addition-cure phenolic resins. Prog. Polym. Sci. 29, 401–498 (2004).
Hopewell, J. L., George, G. A. & Hill, D. J. T. Quantitative analysis of bismaleimide–diamine thermosets using near infrared spectroscopy. Polymer (Guildf) 41, 8221–8229 (2000).
Chaplin, A., Hamerton, I., Herman, H., Mudhar, A. K. & Shaw, S. J. Studying water uptake effect in resins based on cyanate ester/bismalemide blends. Polymer (Guildf) 41, 3945–3956 (2000).
King, J. J., Chaudhari, M. & Zahir, S. A new bismaleimide system for high performance applications. 29th SAMPE Symposium 29, 392–403 (1984).
Morgan, R. J., Shin, E. E., Rosenberg, B. & Jurek, A. Characterization of the cure reactions of bismaleimide composite matrices. Polymer (Guildf) 38, 639–646 (1997).
Rozenberg, B. A., Dzhavadyan, E. A., Morgan, R. & Shin, E. High-performance bismaleimide matrices: cure kinetics and mechanism. Polym. Adv. Technol. 13, 837–844 (2002).
Hirayama, K., Irie, T., Teramoto, N. & Shibata, M. High performance bio-based thermosetting resins composed of dehydrated castor oil and bismaleimide. J. Appl. Polym. Sci. 114, 1033–1039 (2009).
Shibata, M., Teramoto, N. & Nakamura, Y. High performance bio-based thermosetting resins composed of tung oil and bismaleimide. J. Appl. Polym. Sci. 119, 896–901 (2011).
Chiellini, E., Chiellini, F. & Cinelli, P. in Degradable Polymers: Principles and Applications ed. Scott G., Ch. 7, 192–194 (Springer, Berlin (2003).
Roy, D. R., Basu, P. K., Raghunathan, P. & Eswaran, S. V. Cashew nut shell liquid-based tailor-made novolac resins: polymer morphology quantitatation by 1-D and 2-D NMR techniques and performance evaluation. J. Appl. Polym. Sci. 89, 1959–1965 (2003).
Yadav, R., Devi, A., Tripathi, G. & Srivastava, D. Optimization of the process variables for the synthesis of cardanol-based novolac-type phenolic resin using response surface methodology. Eur. Polym. J. 43, 3531–3537 (2007).
Santos, R. S. S., Souza, A. A., Paoli, M. A. & Souza, C. M. L. Cardanol–formaldehyde thermoset composites reinforced with buriti fibers: preparation and characterization. Composites Part A 41, 1123–1129 (2010).
Devi, A. & Srivastava, D. Studies on the blends of cardanol-based epoxidized novolac type phenolic resin and carboxyl-terminated polybutadiene (CTPB), I. Mater. Sci. Eng. A 458, 336–347 (2007).
Kim, Y. H., An, E. S., Park, S. Y. & Song, B. K. Enzymatic epoxidation and polymerization of cardanol obtained from a renewable resource and curing of epoxide-containing polycardanol. J. Mol. Catal. B 45, 39–44 (2007).
Campaner, P., D’Amico, D., Longo, L., Stifani, C. & Tarzia, A. Cardanol-based novolac resins as curing agents of epoxy resins. J. Appl. Polym. Sci. 114, 3585–3591 (2009).
Yadav, R. & Srivastava, D. Synthesis and properties of cardanol-based epoxidized novolac resins modified with carboxyl-terminated butadiene–acrrylonitrile copolymer. J. Appl. Polym. Sci. 114, 1670–1681 (2009).
Bai, W., Xiao, X., Chen, Q., Xu, Y., Zheng, S. & Lin, J. Synthesis and characterization of cross-linked polymer from cardanol by solvent-free grinding polymerization. Prog. Org. Coat. 75, 184–189 (2012).
Lu, Y., Sun, W. & Shen, Z. Polymerization of N-phenylmaleimide with a rare-earth coordination catalyst. J. Appl. Polym. Sci. 96, 979–982 (2005).
Silverstein, R. M., Webster, F. X. & Kiemle, D. J. Spectrometric Identification of Organic Compounds. 7th edn, Vol. 125 (Wiley, Hoboken, (2005).
Shibahara, S., Yamamoto, T., Yamaji, T., Motoyoshiya, J. & Hayashi, S. Thermal reactions of N-phenylmaleimide and mono- or di-functional allylphenols. Polym. J. 30, 404–409 (1998).
Acknowledgements
We thank Dr Naozumi Teramoto of our department for the helpful suggestions and measuring 1H-NMR spectra. We are also grateful to Mr Ryusuke Osada of Material Analysis Center of our university for assisting in FE-SEM measurements.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Shibata, M., Itakura, Y. & Watanabe, H. Bio-based thermosetting resins composed of cardanol novolac and bismaleimide. Polym J 45, 758–765 (2013). https://doi.org/10.1038/pj.2012.195
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2012.195
Keywords
This article is cited by
-
Production of epoxidized cardanol–based vinyl ester resins with cinnamic acid for eco-friendly coating materials
Emergent Materials (2022)
-
Synthesis and properties of phosphorus-containing cardanol-based acrylates for flame-retardant UV/EB-cured coatings
Journal of Coatings Technology and Research (2021)
-
Synthesis and properties of UV-curable cardanol-based acrylate oligomers with cyclotriphosphazene core
Journal of Coatings Technology and Research (2019)
-
Cardanol Polymerization Under Acid Conditions By Addition And Condensation Reactions
Journal of Polymers and the Environment (2018)
-
Green polymer chemistry: the biomimetic oxidative polymerization of cardanol for a synthetic approach to ‘artificial urushi’
Polymer Journal (2017)