Abstract
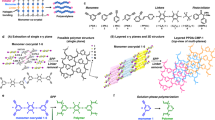
For high-performance boron neutron capture therapy (BNCT), core-shell-type biodegradable nanoparticles possessing boron clusters in their cores are designed to improve their accumulation tendency in the tumor region. The copolymerization of styrene carrying carborane with a poly(ethylene glycol)-block-poly(lactide) copolymer (PEG-b-PLA) that possesses an acetal group at its PEG end and a methacryloyl group at its PLA end (acetal-PEG-b-PLA-MA) was performed in an aqueous medium. As the acetal-PEG-b-PLA-MA forms a polymeric micelle in an aqueous medium, the carborane monomer in the hydrophobic core is solubilized, and the carborane in the core of the micelle is given covalent conjunction. Two carborane compounds, carborane with a mono-vinylbenzyl group [1-(4-vinylbenzyl)-closo-carborane (VB-carborane)] and carborane with di-vinylbenzyl groups [1,2-bis(4-vinylbenzyl)-closo-carborane ((VB)2-carborane)], were newly prepared and employed for the core polymerizations. The encapsulation efficiency of the VB-carborane in the micelles is much higher than that of the (VB)2-carborane. This improved efficiency is most likely caused by the interaction of the hydrogen bonding of the VB-carborane with the polymer micelle matrix, while no hydrogen bonding sites remain in the case of the (VB)2-carborane. The obtained core-polymerized and boron-conjugated micelles (PM, which were prepared from VB-carborane) showed extremely high stability under physiological conditions, which suppressed the leakage of boron compounds owing to the existence of the covalent bonds. The accumulation of the PM (7.2 ID% per g) in tumors is much higher than that of the non-polymerized micelles (NPM) (0.7 ID% per g) under the same conditions (at 24 h after injection) because of the enhanced stability of the micelles under physiological conditions. In addition, thermal neutron irradiation experiments indicated significant suppression of the growth of tumor volume in tumor-bearing mice treated with the PM. It is important to note that the PM administered via intravenous injection were excreted almost completely from the major organs, except for the tumor, after a week. Therefore, these boron-conjugated micelles represent a promising approach to the creation of a novel boron carrier for BNCT.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barth, R. F., Soloway, A. H., Fairchild, R. G. & Brugger, R. M. Boron neutron capture therapy of cancer: Realities and prospects. Cancer 70, 2995–3007 (1992).
Barth, R. F., Coderre, J. A., Vincente, G. H. & Blue, T. E. Boron neutron capture therapy of cancer: Current status and future property. Cli. Cancer Res. 11, 3987–4002 (2005).
Locher, G. L. Biological effects and therapeutic possibilities of neutrons. Am. J. Roentgenol Radium Ther. 36, 1–13 (1936).
Farr, L. E., Sweet, W. H., Robertson, J. S., Foster, C. G., Locksley, H. B., Sutherland, D. L., Mendelsohn, M. L. & Stickley, E. E. Neutron capture therapy with boron in the treatment of glioblastoma multiforme. Am. J. Roentgenol Radium Ther. Nucl. Med. 71, 279–291 (1954).
Godwin, J. T., Farr, L. E., Sweet, W. H. & Robertson, J. S. Pathological study of eight patients with glioblastoma multiforme treated by neutroncapture therapy using boron-10. Cancer 8, 601–615 (1955).
Asbury, A. K., Ojemann, Nielson, S. L. & Sweet, W. H. Neuropathological study of fourteen cases of malignant brain tumor treated by boron-10 slow neutron capture therapy. J. Neuropathol Exp. Neurol. 31, 278–303 (1972).
Knoth, W. H., Sauer, J. C., England, D. C., Hertler, W. R. & Murtterties, E. L. Chemistry of boranes. XIX. derivative chemistry of B10H10-2 and B12H12-2. J. Am. Chem. Soc. 86, 3973–3983 (1964).
Snyder, H. R., Reedy, A. J. & Lennarz, W. Synthesis of aromatic boronic acids. Aldehyde boronic acids and a boronic acid analog of tyrosine. J. Am. Chem. Soc. 80, 835–838 (1958).
Roberts, D. C., Suda, K., Samanen, J. & Kemp, D. S. Pluripotential amino acids. 1. (L)-p-dihydroxyborylphenylalanine (L-Bph) as a precursor of the L-Phe and L-Tyr containing peptides; specific tritiation of L-Phe containing peptides as a final step in synthesis. Tetrahedron Lett. 21, 3435–3438 (1989).
Soloway, A. H., Hatanaka, H. & Davis, M. A. Penetration of brain and brain tumor. VII. Tumor-binding sulfhydryl boron compounds. J. Med. Chem. 10, 714–717 (1967).
Hatanaka, H. A revised boron-neutron capture therapy for malignant brain tumors: II. Interim clinical result with the patients excluding previous treatments. J. Neurol. 209, 81–94 (1975).
Hatanaka, H. & Nakagawa, Y. Clinical results of long-surviving brain tumor patients who underwent boron neutron capture therapy. Int. J. Radiat. Oncol. Biol. Phys. 28, 1061–1066 (1994).
Mishima, Y., Ichihashi, M., Hatta, S., Honda, C., Yamaura, K. & Nakagawa, T. New thermal neutron capture therapy for malignant melanoma: melanogenesis-seeking 10B molecule-melanoma cell interaction from in vitro to first clinical trial. Pigment. Cell. Res. 2, 226–234 (1989).
Gibson, C. R., Staubus, A. E., Barth, R. F., Yang, W., Kleinholz, N. M., Jones, R. B., Church, K. B. G., Tjarks, W. & Soloway, A. H. Electrospray ionization mass spectrometry coupled to reversed-phase ion-pair high-performance liquid chromatography for quantitation of sodium borocaptate and application to pharmacokinetic analysis. Anal. Chem. 74, 2394–2399 (2002).
Ichikawa, H., Taniguchi, E., Fujimoto, T. & Fukumori, Y. Biodistribution of BPA and BSH after single, repeated and simultaneous administrations for neutron-capture therapy of cancer. Appl. Radiat. Isot. 67, 111–114 (2009).
Wu, G. 1., Barth, R. F., Yang, W., Lee, R., Tjarks, W., Backer, M. V. & Backer, J. M. Boron containing macromolecules and nanovehicles as delivery agents for neutron capture therapy. Anticancer Agents Med. Chem. 6, 167–170 (2006).
Yanagie, H., Ogata, A., Sugiyama, H., Eriguchi, M., Takamoto, S. & Takahashi, H. Application of drug delivery system to boron neutron capture therapy for cancer. Expert Opin. Drug. Deliv. 5, 427–443 (2008).
Matsumura, Y. & Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer. Res. 46, 6387–6392 (1986).
Maeda, H., Sawa, T. & Konno, T. Mechanism of tumor-targeted delivery of macromolecular drugs, including the EPR effect in solid tumor and clinical overview of the prototype polymeric drug SMANCS. J. Control Release. 74, 47–61 (2001).
Barth, R. F., Adams, D. M., Soloway, A. H., Alam, F. & Darby, M. V. Boronated starburst dendrimer-monoclonal antibody immunoconjugates: Evaluation as a potential delivery system for neutron capture therapy. Bioconjug Chem. 5, 58–66 (1994).
Wu, G., Barth, R. F., Yang, W., Chatterjee, M., Tjarks, W., Ciesielski, M. J. & Fenstermaker, R. A. Site-specific conjugation of boron-containing dendrimers to anti-EGF receptor monoclonal antibody cetuximab (IMC-C225) and its evaluation as a potential delivery agent for neutron capture therapy. Bioconjug. Chem. 15, 185–194 (2004).
Gedda, L., Olsson, P., Ponten, J. & Carlsson, J. Development and in vitro studies of epidermal growth factor-dextran conjugates for boron neutron capture therapy. Bioconjug. Chem. 7, 584–591 (1996).
Meo, C. D., Panza, L., Capitani, D., Mannia, L., Banzato, A., Rondina, M., Renier, D., Rosato, A. & Crescenzi, V. Hyaluronan as Carrier of carboranes for tumor targeting in boron neutron capture therapy. Biomacromolecules 8, 552–559 (2007).
Mehta, S. C., Lai, J. C. K. & Lu, D. R. Liposomal formulations containing sodium mercaptoundecahydrododecaborate (BSH) for boron neutron capture therapy. J. Microencapsul. 13, 269–279 (1996).
Ishida, O., Maruyama, K., Tanahashi, H., Iwatsuru, M., Sasaki, K., Eriguchi, M. & Yanagie, H. Liposomes bearing polyethyleneglycol-coupled transferrin with intracellular targeting property to the solid tumors in vivo. Pharm. Res. 18, 1042–1048 (2001).
Maruyama, K., Ishida, O., Kasaoka, S., Takizawa, T., Utoguchi, N., Shinohara, M., Chiba, M., Kobayashi, H., Eriguchi, M. & Yanagie, H. Intracellular targeting of sodium mercaptoundecahydrododecaborate (BSH) to solid tumors by transferring-PEG liposomes, for boron neutron-capture therapy (BNCT). J. Control. Release. 98, 195–207 (2004).
Yanagie, H., Maruyama, K., Takizawa, T., Ishida, O., Ogura, K., Matsumoto, T., Sakurai, Y., Kobayashi, T., Shinohara, A., Rant, J., Skvarc, J., Ilic, R., Kuhne, G., Chiba, M., Furuya, Y., Sugiyama, H., Hisa, T., Ono, K., Kobayashi, H. & Eriguchi, M. Application of boron-entrapped stealth liposomes to inhibition of growth of tumour cells in the in vivo boron neutron-capture therapy model. Biomed. Pharmacother. 60, 43–50 (2006).
Wagner, E., Curiel, D. & Cotton, M. Delivery of drugs, proteins and genes into cells using transferring as a ligand for receptor-mediated endocytosis. Adv. Drug. Deliv. Rev. 14, 113–135 (1994).
Feakes, D. A., Shelly, K. & Hawthorne, M. F. Selective boron delivery to murine tumors by lipophilic species incorporated in the membranes of unilamellar liposomes. Proc. Natl. Acad. Sci. USA 92, 1367–1370 (1995).
Miyajima, Y., Nakamura, H., Kuwata, Y., Lee, J. D., Masunaga, S., Ono, K. & Maruyama, K. Transferrin-loaded nido-carborane liposomes: tumor-targeting boron delivery system for neutron capture therapy. Bioconjug. Chem. 17, 1314–1320 (2006).
Li, T., Hamdi, J. & Hawthorne, M. F. Unilamellar liposomes with enhanced boron content. Bioconjug. Chem. 17, 15–20 (2006).
Lee, J. D., Ueno, M., Miyajima, Y. & Nakamura, H. Synthesis of boron cluster lipids: closo-dodecaborate as an alternative hydrophilic function of boronated liposomes for neutron capture therapy. Org. Lett. 9, 323–326 (2007).
Ueno, M., Ban, H. S., Nakai, K., Inomata, R., Kaneda, Y., Matsumura, A. & Nakamura, H. Dodecaborate lipid liposomes as new vehicles for boron delivery system of neutron capture therapy. Bioorg. Med. Chem. 18, 3059–3065 (2010).
Kataoka, K., Harada, A. & Nagasaki, Y. Block copolymer micelles for drug delivery: design characterization and biological significance. Adv. Drug. Dliv. Rev. 47, 113–131 (2001).
Gaucher, G., Dufresne, M. H., Sant, V. P., Kang, N., Maysinger, D. & Leroux, J. C. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control Release 109, 169–199 (2005).
Verrechia, T., Spenlehauer, G., Bazile, D. V., Murry-Brelier, A., Archimbaud, Y. & Veillard, M. Non-stealth (poly(lactic acid/albumin)) and stealth (poly(lactic acid-polyethylene glycol)) nanoparticles as injectable drug carriers. J. Control. Release 36, 49–61 (1995).
Hagan, S. A., Coombes, A. G. A., Garnett, M. C., Dunn, S. E., Davies, M. C., Illum, L., Davis, S. S., Harding, S. E., Purkiss, S. & Gellert, P. R. Polylactide-poly(ethylene glycol) copolymers as drug delivery systems. 1. characterization of water dispersible micelle-forming systems. Langmuir. 12, 2153–2161 (1996).
Yamamoto, Y., Nagasaki, Y., Kato, Y., Sugiyama, Y. & Kataoka, K. Long-circulating poly(ethylene glycol)-poly(D,L-lactide) block copolymer micelles with modulated surface charge. J. Control. Release. 77, 27–38 (2001).
Stefani, M., Coudane, J. & Vert, M. In vitro ageing and degradation of PEG-PLA diblock copolymer-based nanoparticles. Polym. Degrad. Stab. 91, 2554–2559 (2006).
Yang, L., Ghzaoui, A. E. & Li, S. In vitro degradation behavior of Poly(lactide)-Poly(ethylene glycol) block copolymer micelles in aqueous solution. Int. J. Pharm. 400, 96–103 (2010).
Kim, S., Shi, Y., Kim, J. Y., Park, K. & Cheng, J. X. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin. Drug. Deliv. 7, 49–62 (2010).
Chen, H., Kim, S., He, W., Wang, H., Low, P. S., Park, K. & Cheng, J. X. Fast release of lipophilic agents from circulation PEG-PDLLA micelles revealed by in vivo förster resonance energy transfer imaging. Langmuir. 24, 5213–5217 (2008).
Burt, H. M., Zhang, X., Toleikis, P., Embree, L. & Hunter, W. L. Development of copolymers of poly(D,L-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf. B. Biointerfaces 16, 161–171 (1999).
Diezi, T. A., Bae, Y. & Kwon, G. S. Enhanced Stability of PEG-block-poly(N-hexyl stearate L-aspartamide) micelles in the presence of serum proteins. Mol. Pharm. 7, 1355–1360 (2010).
Rösler, A., Vandermeulen, G. W. & Klok, H. A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug. Deliv. Rev. 53, 95–108 (2001).
O’Reilly, R. K., Hawker, C. J. & Wooley, K. L. Cross-linked block copolymer micelles: functional nanostructures of great potential and versatility. Chem. Soc. Rev. 35, 1068–1083 (2006).
Shuai, X., Merdan, T., Schaper, A. K., Xi, F. & Kissel, T. Core-cross-linked polymeric micelles as paclitaxel carriers. Bioconjug. Chem. 15, 441–448 (2004).
Rijcken, C. J., Snel, C. J., Schiffelers, R. M., Nostrum, C. F. & Hennink, W. E. Hydrolysable core-crosslinked thermosensitive polymeric micelles: synthesis, characterization and in vivo studies. Biomaterials 28, 5581–5593 (2007).
Iijima, M., Nagasaki, Y., Okada, T., Kato, M. & Kataoka, K. Core-polymerized reactive micelles from heterotelechelic amphiphilic block copolymers. Macromolecules 32, 1140–1146 (1999).
Kim, J. H., Emoto, K., Iijima, M., Nagasaki, Y., Aoyagi, T., Okano, T., Sakurai, Y. & Kataoka, K. Core-stabilized polymeric micelle as potential drug carrier: increased solubilization of taxol. Polym. Adv. Technol. 10, 647–654 (1999).
Du, W., Nyström, A. M., Zhang, K., Leonard, J. R. & Wooley, K. L. 19F- and fluorescently labeled micelles as nanoscopic assemblies for chemotherapeutic delivery. Bioconjug. Chem. 19, 2492–2498 (2008).
Yokoyama, M., Okano, T., Sakurai, Y., Ekimoto, H., Shibazaki, C. & Kataoka, K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 51, 3229–3236 (1991).
Yoo, H. S., Lee, E. A. & Park, T. G. Doxorubicin-conjugated biodegradable polymeric micelles having acid-cleavable linkage. J. Control Release 82, 17–27 (2002).
Bae, Y., Fukushima, S., Harada, A. & Kataoka, K. Design of environment-sensitive supramolecular assemblies for intercellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 42, 4640–4643 (2003).
Kataoka, K., Matsumoto, T., Yokoyama, M., Okano, T., Sakurai, Y., Fukushima, S., Okamoto, K. & Kwon, G. S. Doxorubicin-loaded poly(ethylene glycol)-poly(β-benzyl-L-aspartate) copolymer micelles: Their pharmaceutical characteristics and biological significance. J. Control Release 64, 143–153 (2000).
Lee, J., Cho, E. C. & Cho, K. Incorporation and release behavior of hydrophobic drug in functionalized poly(D,L-lactide)-block-poly(ethylene oxide) micelles. J. Control Release 94, 323–335 (2004).
Danquah, M., Fujiwara, T. & Mahato, R. I. Self-assembling Methoxypoly(ethylene glycol)-b-poly(carbonate-co-L-lactide) block copolymer for drug delivery. Biomaterials 31, 2358–2370 (2010).
Benhabbour, S. R., Parott, M. C., Gratton, E. A. & Adronov, A. Synthesis and properties of carborane-containing dendronized polymers. Macromolecules 40, 5678–5688 (2007).
Sumitani, S., Oishi, M. & Nagasaki, Y. Carborane confined nanoparticles for boron neutron capture therapy: improved stability, blood circulation time and tumor accumulation. React Funct. Polym. 71, 684–693 (2011).
Davidson, M. G., Hibbert, T. G., Howard, J. A. K., Mackinnon, A. & Wade, K. Definitive crystal structures of ortho-, meta- and para-carboranes: supramolecular structures directed solely by C-H…O hydrogen bonding to hmpa (hmpa=hexamethylphosphoramide). Chem. Commun. 19, 2285–2286 (1996).
Sumitani, S., Oishi, M., Yaguchi, T., Murotani, H., Horiguchi, Y., Suzuki, M., Ono, K., Yanagie, H. & Nagasaki, Y. Pharmacokinetics of core-polymerized, boron-conjugated micelles designed for boron neutron capture therapy for cancer. Biomaterials 33, 3568–3577 (2012).
Lewinski, N., Colvin, V. & Drezek, R. Cytotoxicity of nanoparticles. Small 4, 26–49 (2008).
Acknowledgements
This research was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘Molecular Soft-Interface Science’ (no. 20106011), from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT). This work was performed using facilities of the Research Reactor Institute, Kyoto University and the Japan Atomic Energy Agency.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sumitani, S., Nagasaki, Y. Boron neutron capture therapy assisted by boron-conjugated nanoparticles. Polym J 44, 522–530 (2012). https://doi.org/10.1038/pj.2012.30
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2012.30
Keywords
This article is cited by
-
The basis and advances in clinical application of boron neutron capture therapy
Radiation Oncology (2021)
-
Student model construction of intelligent teaching system based on Bayesian network
Personal and Ubiquitous Computing (2020)
-
Synthesis and biological evaluation of a new series of ortho-carboranyl biphenyloxime derivatives
Chemistry Central Journal (2018)
-
Immobilization of carborane derivatives on Ni/Fe nanotubes for BNCT
Journal of Nanoparticle Research (2018)
-
Silica nanoparticles carrying boron-containing polymer brushes
Journal of Nanoparticle Research (2014)