Abstract
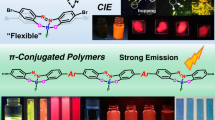
π-Conjugated polymers are synthesized by C–H and C–X polycondensation of 2-(4-haloaryl)thiophene monomers with a nickel catalyst, and the Knochel–Hauser base (TMPMgCl·LiCl (chloromagnesium 2,2,6,6-tetramethylpiperidide lithium chloride salt)). The C–H functionalization polycondensation reaction of a monobrominated monomer undergoes dehydrobrominative polymerization with an equimolar amount of TMPMgCl·LiCl and a catalytic amount of NiCl2dppp to produce poly(2,5-thienylene-1,4-phenylene) and poly(2,5-thienylenepyridine-2,5-diyl) with reasonable yields. Polycondensation with triflate as a leaving group proceeds under similar conditions to produce poly(thienylenephenylene) with an excellent yield. Poly(benzodithiophene) was also obtained from the reaction of the corresponding monobrominated benzodithiophene in the presence of a nickel-catalyst-bearing N-heterocyclic carbene as a ligand.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sirringhaus, H., Brown, P. J., Friend, R. H., Nielsen, M. M., Bechgaard, K., Langeveld-Voss, B. M. W., Spiering, A. J. H., Janssen, R. A. J., Meijer, W. W., Herwig, P. & deLeeuw, D. M. Two-dimensional charge transport in self-organized, high-mobility conjugated polymers. Nature 401, 685–688 (1999).
Beaujuge, P. M. & Fréchet, M. J. Molecular design and ordering effects in π-functional materials for transistor and solar cell applications. J. Am. Chem. Soc. 133, 20009–20029 (2011).
Grimsdale, A. C., Chan, K. L., Martin, R. E., Jokisz, P. G. & Holmes, A. B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 197, 897–1091 (2009).
Yamamoto, T. Synthesis of π-conjugated polymers by organometallic polycondensation. Bull. Chem. Soc. Jpn 83, 431–455 (2010).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Liversedge, I. A., Higgins, S. J., Giles, M., Heeney, M. & McCulloch, I. Suzuki route to regioregular polyalkylthiophenes using Ir-catalysed borylation to make the monomer, and Pd complexes of bulky phosphanes as coupling catalysts for polymerization. Tetrahedron Lett 47, 5143–5146 (2006).
Guillerez, S. & Biden, G. New convenient synthesis of highly regioregular poly(3-octylthiophene) based on the Suzuki coupling reaction. Synth. Met 93, 123–126 (1998).
Iraqi, A. & Barker, G. Synthesis and characterisation of telechelic regioregular head-to-tail poly(3-alkylthiophenes). J. Mater. Chem. 8, 25–29 (1998).
Carsten, B., He, F., Son, H.-J., Xu, T. & Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 111, 1493–1528 (2011).
Yamamoto, T., Zhou, Z.-H., Kanbara, T., Shimura, M., Kizu, K., Maruyama, T., Nakamura, Y., Fukuda, T., Lee, B.-L., Ooba, N., Tomaru, S., Kurihara, T., Kaino, T., Kubota, K. & Sasaki, S. π-Conjugated donor-acceptor copolymers constituted of π-excessive and π-deficient arylene units. Optical and electrochemical properties in relation to CT structure of the polymer. J. Am. Chem. Soc 118, 10389–10399 (1996).
Osaka, I. & McCullough, R. D. Advances in molecular design and synthesis of regioregular polythiophenes. Acc. Chem. Res. 41, 1202–1214 (2008).
Loewe, R. S., Khersonsky, S. M. & McCullough, R. D. A Simple method to prepare head-to-Tail coupled, regioregular poly(3-alkylthiophenes) using Grignard metathesis. Adv. Mater. 11, 250–253 (1999).
Yokoyama, A., Miyakoshi, R. & Yokozawa, T. Chain-growth polymerization for poly(3-hexylthiophene) with a defined molecular weight and a low polydispersity. Macromolecules 37, 1169–1171 (2004).
Sheina, E. E., Liu, J., Iovu, M. C., Laird, D. W. & McCullough, R. D. Chain growth mechanism for regioregular nickel-initiated cross-coupling polymerizations. Macromolecules 37, 3526–3528 (2004).
Miyakoshi, R., Yokoyama, A. & Yokozawa, T. Catalyst-transfer polycondensation. mechanism of Ni-catalyzed chain-growth polymerization leading to well-defined poly(3-hexylthiophene). J. Am. Chem. Soc. 127, 17542–17547 (2005).
Fukumoto, H., Fujiwara, Y. & Yamamoto, T. Preparation of head-to-tail π-conjugated poly(thiophene−pyridine) and polypyrimidine by organometallic polycondensation. Chem. Lett 40, 992–994 (2011).
Wang, Q., Takita, R., Kikuzaki, T. & Ozawa, F. Palladium-catalyzed dehydrohalogenative polycondensation of 2-bromo-3-hexylthiophene: An efficient approach to head-to-tail poly(3-hexylthiophene). J. Am. Chem. Soc. 132, 11420–11421 (2010).
Lu, W., Kuwabara, J. & Kanbara, T. Polycondensation of dibromofluorene analogues with tetrafluorobenzene via direct arylation. Macromolecules 44, 1252–1255 (2011).
Fujinami, Y., Kuwabara, J., Lu, W., Hayashi, H. & Kanbara, T. Synthesis of thiophene- and bithiophene-based alternating copolymers via Pd-catalyzed direct C−H arylation. ACS Macro. Lett. 1, 67–70 (2012).
Liégault, B., Lapointe, D., Caron, L., Vlassova, A. & Fagnou, K. Establishment of broadly applicable reaction conditions for the palladium-catalyzed direct arylation of heteroatom-containing aromatic compounds. J. Org. Chem. 74, 1826–1834 (2009).
Krasovskiy, A., Krasovskaya, V. & Knochel, P. Mixed Mg/Li amides of the type R2NMgCl⋅LiCl as highly efficient bases for the regioselective generation of functionalized aryl and heteroaryl magnesium compounds. Angew. Chem. Int. Ed 45, 2958–2961 (2006).
Hauser, C. R. & Walker, H. G. Condensation of Certain Esters by Means of Diethylaminomagnesium Bromide. J. Am. Chem. Soc 69, 295–297 (1947).
Tamba, S., Tanaka, S., Okubo, Y., Okamoto, S., Meguro, H. & Mori, A. Nickel-catalyzed dehydrobrominative polycondensation for the practical preparation of regioregular poly(3-substituted thiophene)s. Chem. Lett. 40, 398–399 (2011).
Tamba, S., Shono, K., Sugie, A. & Mori, A. C−H functionalization polycondensation of chlorothiophenes in the presence of nickel catalyst with stoichiometric or catalytically-generated magnesium amide. J. Am. Chem. Soc 133, 9700–9703 (2011).
Tanaka, S., Tamba, S., Tanaka, D., Sugie, A. & Mori, A. Synthesis of well-defined head-to-tail-type oligothiophenes by regioselective deprotonation of 3-substituted thiophenes and nickel-catalyzed cross-coupling reaction. J. Am. Chem. Soc 133, 16734–16737 (2011).
Liang, Y., Feng, D., Wu, Y., Tsai, S.-T., Li, G., Ray, C. & Yu, L. Highly efficient solar cell polymers developed via fine-tuning of structural and electronic properties. J. Am. Chem. Soc. 131, 7792–7799 (2009).
Ono, R. J., Kang, S. & Bielawski, C. W. Controlled chain-growth Kumada catalyst transfer polycondensation of a conjugated alternating copolymer. Macromol. doi:10.1021/ma300013e 45, 2321–2326 (2012).
Do, H-Q. & Daugulis, O. An aromatic Glaser−Hay reaction. J. Am. Chem. Soc. 131, 17052–17053 (2009).
Booth, G. & Chatt, J. Some complexes of ditertiary phosphines with nickel(II) and nickel(III). J. Chem. Soc 3238–3241 (1965).
Kwong, F. Y., Lai, C. W., Yu, M., Tian, Y. & Chan, K. S. Palladium-catalyzed phosphination of functionalized aryl triflates. Tetrahedron 59, 10295–10305 (2003).
Hou, J., Park, M.-H., Zhang, S., Yao, Y., Chen, L.-M., Li, J.-H. & Yang, Y. Bandgap and molecular energy level control of conjugated polymer photovoltaic materials based on benzo[1,2-b:4,5-b’]dithiophene. Macromolecules 41, 6012–6018 (2008).
Kim, D. W., Choi, H. Y., Lee, K.-J. & Chi, D. Y. Facile oxidation of fused 1,4-dimethoxybenzenes to 1,4-quinones using NBS: Fine-tuned control over bromination and oxidation reactions. Org. Lett. 3, 445–447 (2001).
O'Brien, C. J., Kantchev, E. A. B., Valente, C., Hadei, N., Chass, G. A., Lough, A., Hopkinson, A. C. & Organ, M. G. Easily prepared air- and moisture-stable Pd–NHC (NHC=N-Heterocyclic Carbene) complexes: A reliable, user-friendly, highly active palladium precatalyst for the Suzuki–Miyaura reaction. Chem. Eur. J 12, 4743–4748 (2006).
Muto, K., Yamaguchi, J. & Itami, K. Nickel-catalyzed C−H/C−O coupling of azoles with phenol derivatives. J. Am. Chem. Soc. 134, 169–172 (2012).
Shiraishi, K. & Yamamoto, T. New π-conjugated polymers constituted of dialkoxybenzodithiophene units: synthesis and electronic properties. Synth. Met 130, 139–147 (2002).
Acknowledgements
This work was partially supported by KAKENHI (B) by the Japan Society for the Promotion of Science (JSPS). Thiophene derivatives were partly donated by Soken Chemical & Engineering Co. Ltd. We thank the Nara Institute of Science and Technology, Kyoto-Advanced Nanotechnology Network, supported by MEXT, Japan, for measurements of high-resolution mass spectra, and Professor Takeshi Shiono of the Hiroshima University for measurement of high-temperature SEC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamba, S., Okubo, Y., Sugie, A. et al. Synthesis of π-conjugated poly(thienylenearylene)s with nickel-catalyzed C–H functionalization polycondensation. Polym J 44, 1209–1213 (2012). https://doi.org/10.1038/pj.2012.89
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2012.89