Abstract
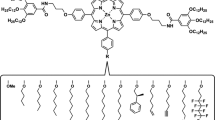
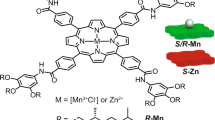
Inspired by functional systems in nature, chemists have created a number of intriguing and useful molecular systems from porphyrins and their metal complexes. Of the synthetic porphyrin derivatives developed to date, strapped porphyrins are unique because they have three-dimensional architectures based on a built-in two-dimensional porphyrin molecule. Consequently, the structures of strapped porphyrins can be customized through detailed molecular design, thereby allowing the synthesis of sophisticated molecular systems. Herein, we describe strapped porphyrin-based polymeric systems. In particular, we focus on molecular design concepts that are established in combination with photophysical, electronic and mechanical properties of polymeric materials.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kadish, K. M., Smith, K. M. & Guilard, R. Handbook of Porphyrin Science, (World Scientific, Singapore, Singapore, 2010).
Kim, D. Multiporphyrin Arrays, Fundamentals and Applications, (Pan Stanford Publishing, Singapore, Singapore, 2012).
Collman, J. P., Zhang, X., Lee, V. J., Uffelman, E. S. & Brauman, J. I. Regioselective and enentioselective epoxidation catalyzed by metalloporphyrins. Science 261, 1404–1411 (1993).
Momenteau, M. & Reed, C. A. Synthetic heme dioxygene complexes. Chem. Rev. 94, 659–698 (1994).
Rose, E., Andrioletti, B., Zrig, S. & Quelquejeu-Tthéve, M. Enentioselective epoxidation of olefins with chiral metalloporphyrin catalysts. Chem. Soc. Rev. 34, 573–583 (2005).
Thordarson, P., Bijsterveld, E. J. A., Rowan, A. E. & Nolte, R. J. M. Epoxidation of polybutadiene by a topologically linked catalyst. Nature 424, 915–918 (2003).
Takeuchi, M., Shioya, T. & Swager, T. M. Allosteric fluoride anion recognition by a doubly strapped porphyrin. Angew. Chem. Int. Ed. 40, 3372–3376 (2001).
Koepf, M., Wytko, J. A., Bucher, J.-P. & Weiss, J. Surface-tuned assembly of porphyrin coordination oligomers. J. Am. Chem. Soc. 130, 9994–10001 (2008).
Takeuchi, M., Fujikoshi, C., Kubo, Y., Kaneko, S. & Shinkai, S. Conjugated polymers complexed with helical porphyrin oligomers create micron-sized ordered structures. Angew. Chem. Int. Ed. 45, 5494–5499 (2006).
Zhang, X. & Takeuchi, M. Controlled fabrication of fullerene C60 into microsphere of nanoplates through porphyrin polymer assisted self-assembly. Angew. Chem. Int. Ed. 48, 9646–9651 (2009).
Ikeda, T., Lintuluoto, J. M., Aratani, N., Yoon, Z. S., Kim, D. & Osuka, A. Synthesis of doubly strapped porphyrin arrays and triply lined conjugated porphyrin tapes. Eur. J. Org. Chem. 3193–3204 (2006).
Frampton, M. J. & Anderson, H. L. Insulated molecular wires. Angew. Chem. Int. Ed. 46, 1028–1064 (2007).
Aratani, N., Kim, D. & Osuka, A. Discrete cyclic porphyrin arrays as artificial light-harvesting antenna. Acc. Chem. Res. 42, 1922–1934 (2009).
Ogi, S., Sugiyasu, K. & Takeuchi, M. Synthesis and fluorescence resonance energy transfer properties of an alternating donor-acceptor copolymer featuring orthogonally arrayed transition dipoles along the polymer backbone. ACS Macro Lett. 1, 1199–1203 (2012).
Ogi, S., Sugiyasu, K. & Takeuchi, M. Synthesis of a doubly strapped light-harvesting porphyrin bearing energy donor molecules hanging on to the straps: an attempt toward macroscopic control over molecular conformation that affects the efficiency of fluorescence resonance energy transfer. Bull. Chem. Soc. Jpn 84, 40–48 (2011).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy 3rd edn (Springer, New York, NY, USA, 2006).
Davis, D. A., Hamilton, A., Yang, J., Cremar, L. D., van Gough, D., Potisek, S. L., Ong, M. T., Braun, P. V., Martínez, T. J., White, S. R., Moore, J. S. & Sottos, N. R. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 459, 68–72 (2009).
Caruso, M. M., Davis, D. A., Shen, Q., Odom, S. A., Sottos, N. R., White, S. R. & Moore, J. S. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Skotheim, T. A. & Reynolds, J. R. Handbook of Conducting Polymers, (CRC Press, Boca Raton, FL, USA, 2007).
Sugiyasu, K. & Takeuchi, M. Conducting polymer networks cross-linked by ‘isolated’ functional dyes: design, synthesis, and electrochemical polymerization of doubly strapped light-harvesting porphyrin/oligothiophene monomers. Chem. Eur. J. 15, 6350–6362 (2009).
Brunsveld, L., Folmer, B. J. B., Meijer, E. W. & Sijbesma, R. P. Supramolecular Polymers. Chem. Rev. 101, 4071–4097 (2001).
De Greef, T. F. A., Smulders, M. M. J., Wolffs, M., Schemming, A. P. H. J., Sijbesma, R. P. & Meijer, E. W. Supramolecular polymerization. Chem. Rev. 109, 5687–5754 (2009).
Aida, T., Meijer, E. W. & Stupp, S. I. Functional supramolecular polymers. Science 335, 813–817 (2012).
Ogi, S., Sugiyasu, K. & Takeuchi, M. Unpublished results.
Ogi, S., Sugiyasu, K., Swarup, M., Samitsu, S. & Takeuchi, M. Living supramolecular polymerization realized through a biomimetic approach. Nat. Chem. 6, 188–195 (2014).
Alexandridis, P. & Lindman, B. Amphiphilic Block Copolymers, Self-Assembly and Applications, (Elsevier Science B.V. Amsterdam, The Netherlands, 2000).
Sugiyasu, K., Honsho, Y., Harrison, R. M., Sato, A., Yasuda, T., Seki, S. & Takeuchi, M. A self-threading polythiophene: defect-free insulated molecular wires endowed with long effective conjugation length. J. Am. Chem. Soc. 132, 14754–14756 (2010).
Shomura, R., Sugiyasu, K., Yasuda, T., Sato, A. & Takeuchi, M. Electrochemical generation and spectroscopic characterization of charge carriers within isolated planar polythiophene. Macromolecules 45, 3759–3771 (2012).
Ouchi, Y., Sugiyasu, K., Ogi, S., Sato, A. & Takeuchi, M. Synthesis of self-threading bithiophenes and their structure-property relationships regarding cyclic side-chains with atomic precision. Chem. Asian J. 7, 75–84 (2012).
Pan, C., Sugiyasu, K., Wakayama, Y., Sato, A. & Takeuchi, M. Thermoplastic fluorescent conjugated polymers: benefits of preventing π–π stacking. Angew. Chem. Int. Ed. 52, 10775–10779 (2013).
Pan, C., Sugiyasu, K., Aimi, J., Sato, A. & Takeuchi, M. Picket fence polythiophene and its diblock copolymers that afford microphase separations comprising a stacked and isolated polythiophene ensemble. Angew. Chem. Int. Ed. doi:10.1002/anie.201402813.
Pan, C., Sugiyasu, K. & Takeuchi, M. Blending conjugated polymers without phase separation for fluorescent colour tuning of polymeric materials through FRET Chem. Commun. (e-pub ahead of print 17 June 2014; doi: 10.1039/C4CC03594A).
Kiguchi, M., Ohto, T., Fujii, S., Sugiyasu, K., Nakajima, S., Takeuchi, M. & Nakamura, H. Single molecular resistive switch obtained via sliding multiple anchoring points and varying effective wire length. J. Am. Chem. Soc. 136, 7327–7332 (2014).
Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI) (Nos. 20750097, 23655108 and 25620101), Grant-in-Aid for Scientific Research on Innovative Areas ‘Dynamical ordering of biomolecular systems for creation of integrated functions’, the Shorai Foundation for Science and Technology, the Association for the Progress of New Chemistry and by the NIMS Molecule and Material Synthesis Platform of the ‘Nanotechnology Platform Project’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugiyasu, K., Ogi, S. & Takeuchi, M. Strapped porphyrin-based polymeric systems. Polym J 46, 674–681 (2014). https://doi.org/10.1038/pj.2014.58
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2014.58
This article is cited by
-
Reversible coordination-induced spin-state switching in complexes on metal surfaces
Nature Nanotechnology (2020)