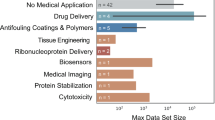
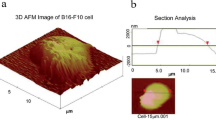
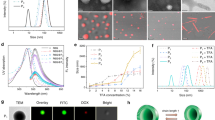
Abstract
Artificial replication of the biomembrane systems in living organisms is attractive for the development of advanced functional materials but remains challenging for materials science because of the intricate function of these systems. To this end, free-standing polymeric ultrathin films (referred to as ‘polymer nanosheets’) have been developed as a structural analog of biomembranes, such as cellular membranes and basement membranes in an extracellular matrix, with a thickness of tens to hundreds of nanometers. In comparison with conventional plastic films, these ultrathin structures generate attractive properties for biomedical applications, including high flexibility and noncovalent adhesiveness. This report reviews the seminal features and characteristics of ‘nanosheet technology’, including fabrication methods, mechanical properties and biomedical and health-care applications (for example, wound dressings, tissue engineering materials and bioelectronic devices). Nanosheet technology is a promising approach for the development of advanced medical applications and health-care practices in surgery and regenerative medicine, as well as for connecting the human body to electronic interfaces for future medical applications.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Whitesides, G. M., Mathias, J. P. & Seto, C. T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science 254, 1312–1319 (1991).
Förster, S. & Plantenberg, T. From self-organizing polymers to nanohybrid and biomaterials. Angew. Chem. Int. Ed. 41, 688–714 (2002).
Ringsdorf, H., Schlarb, B. & Venzmer, J. Molecular architecture and function of polymeric oriented systems: models for the study of organization, surface recognition, and dynamics of biomembranes. Angew. Chem. Int. Ed. 27, 113–158 (1988).
Kunitake, T. Ultrathin films as biomimetic membranes. Polym. J. 23, 613–618 (1991).
Costa, R. R. & Mano, J. F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 43, 3453–3479 (2014).
Pérez-Madrigal, M. M., Armelin, E., Puiggalí, J. & Alemán, C. Insulating and semiconducting polymeric free-standing nanomembranes with biomedical applications. J. Mater. Chem. B 3, 5904–5932 (2015).
Fujie, T., Okamura, Y. & Takeoka, S. in Functional Polymer Films (eds Knoll, W. & Advincula, R. C.) Ch. 29, 907–931 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011).
Fujie, T. & Takeoka, S. in Nanobiotechnology (eds Phoenix, D. A. & Waqar, A.) Ch. 6, 68–94 (One Central Press, UK, 2014).
Mamedov, A. A. & Kotov., N. A. Free-standing layer-by-layer assembled films of magnetite nanoparticles. Langmuir 16, 5530–5533 (2000).
Forrest, J. A., Dalnoki-Veress, K., Stevens, J. R. & Dutcher, J. R. Effect of free surfaces on the glass transition temperature of thin polymer films. Phys. Rev. Lett. 77, 2002–2005 (1996).
Jiang, C. & Tsukruk, V. V. Freestanding nanostructures via Layer-by-Layer assembly. Adv. Mater. 18, 829–840 (2006).
Endo, H., Kado, Y., Mitsuishi, M. & Miyashita, T. Fabrication of free-standing hybrid nanosheets organized with polymer Langmuir−Blodgett films and gold nanoparticles. Macromolecules 39, 5559–5563 (2006).
Vendamme, R., Onoue, S., Nakao, A. & Kunitake, T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat. Mater. 5, 494–501 (2006).
Liao, I., Wan, A. C. A., Yim, E. K. F. & Leong, K. W. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release 104, 347–358 (2005).
Kumar, M. N., Muzzarelli, R. A., Muzzarelli, C., Sashiwa, H. & Domb, A. J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104, 6017–6084 (2004).
Baba, S., Midorikawa, T. & Nakano, T. Unambiguous detection of the adhesive failure of metal films in the microscratch test by waveform analysis of the friction signal. Appl. Surf. Sci. 144, 344–349 (1999).
Vlassak, J. J. & Nix, W. D. A new bulge test technique for the determination of Young's modulus and Poisson's ratio of thin films. J. Mater. Res. 7, 3242–3249 (1992).
Markutsya, S., Jiang, C., Pikus, Y. & Tsukruk., V. V. Freely suspended layer-by-layer nanomembranes: testing micromechanical properties. Adv. Funct. Mater. 15, 771–780 (2005).
Stafford, C. M., Harrison, C., Beers, K. L., Karim, A., Amis, E. J., Vanlandingham, M. R., Kim, H.-C., Volksen, W., Miller, R. D. & Simonyi, E. E. A buckling-based metrology for measuring the elastic moduli of polymeric thin films. Nat. Mater. 3, 545–550 (2004).
Fujie, T., Kawamoto, Y., Haniuda, H., Saito, A., Kabata, K., Honda, Y., Ohmori, E., Asahi, T. & Takeoka, S. Selective molecular permeability induced by glass transition dynamics of semi-crystalline polymer ultra-thin films. Macromolecules 46, 395–402 (2013).
Eling, B., Gogolewski, S. & Pennings, A. J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 23, 1587–1593 (1982).
Porte, H. L., Jany, T., Akkad, R., Conti, M., Gillet, P. A., Guidat, A. & Wurtz, A. J. Randomized controlled trial of a synthetic sealant for preventing alveolar air leaks after lobectomy. Ann. Thorac. Surg. 71, 1618–1622 (2001).
Kawamura, M., Gika, M., Izumi, Y., Horinouchi, H., Shinya, N., Mukai, M. & Kobayashi, K. The sealing effect of fibrin glue against alveolar air leakage evaluated up to 48h; comparison between different methods of application. Eur. J. Cardiothorac. Surg. 28, 39–42 (2005).
Gika, M., Kawamura, M., Izumi, Y. & Kobayashi, K. The short-term efficacy of fibrin glue combined with absorptive sheet material in visceral pleural defect repair. Interact. Cardiovasc. Thorac. Surg. 6, 12–15 (2007).
Fujie, T., Okamura, Y. & Takeoka, S. Ubiquitous transference of free-standing polysaccharide nanosheet in the development of a nano-adhesive plaster. Adv. Mater. 19, 3549–3553 (2007).
Fujie, T., Matsutani, N., Kinoshita, M., Okamura, Y., Saito, A. & Takeoka, S. Adhesive, flexible and robust polysaccharide nanosheet integrated for tissue-defect repair. Adv. Funct. Mater. 19, 2560–2568 (2009).
Malangoni, M. A. Contributions to the management of intraabdominal infections. Am. J. Surg. 190, 255–259 (2005).
Brook, I. Microbiology and management of abdominal infections. Dig. Dis. Sci. 53, 2585–2591 (2008).
Fujie, T., Saito, A., Kinoshita, M., Miyazaki, H., Ohtsubo, S., Saitoh, D. & Takeoka, S. Dual therapeutic action of antibiotic-loaded nanosheets for the treatment of gastrointestinal tissue defects. Biomaterials 31, 6269–6278 (2010).
Saito, A., Miyazaki, H., Fujie, T., Ohtsubo, S., Kinoshita, M., Saitoh, D. & Takeoka, S. Therapeutic efficacy of an antibiotic-loaded nanosheet in a murine burn-wound infection model. Acta Biomater. 8, 2932–2940 (2012).
Ito, K., Saito, A., Fujie, T., Nishiwaki, K., Miyazaki, H., Kinoshita, M., Saitoh, D., Ohtsubo, S. & Takeoka, S. Sustainable antimicrobial effect of silver sulfadiazine-loaded nanosheets on infection in a mouse model of partial-thickness burn injury. Acta Biomater. 24, 87–95 (2015).
Ostrovidov, S., Hosseini, V., Ahadian, S., Fujie, T., Prakash Parthiban, S., Ramalingam, M., Bae, H., Kaji, H. & Khademhosseini, A. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 20, 403–436 (2014).
Ghaemmaghami, A. M., Hancock, M. J., Harrington, H., Kaji, H. & Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 17, 173–181 (2012).
Ricotti, L. & Menciassi, A. Engineering stem cells for future medicine. IEEE Trans. Biomed. Eng. 60, 727–734 (2013).
Khademhosseini, A., Langer, R., Borenstein, J. T. & Vacanti, J. P. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA 103, 2480–2487 (2006).
Hollister, S. J. Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524 (2005).
Fu, C., Grimes, A., Long, M., Ferri, C. G. L., Rich, B. D., Ghosh, S., Ghosh, S., Lee, L. P., Gopinathan, A. & Khine, M. Tunable nanowrinkles on shape memory polymer sheets. Adv. Mater. 21, 4472–4476 (2009).
Liang, D., Hsiao, B. S. & Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 59, 1392–1412 (2007).
Schuurman, W., Khristov, V., Pot, M. W., van Weeren, P. R., Dhert, W. J. A. & Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3, 021001 (2011).
Tawfick, S., De Volder, M., Copic, D., Park, S.-J., Oliver, C. R., Polsen, E. S., Roberts, M. J. & Hart, A. J. Engineering of micro- and nanostructured surfaces with anisotropic geometries and properties. Adv. Mater. 24, 1628–1674 (2012).
Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011).
Fujie, T., Ahadian, S., Liu, H., Chang, H., Ostrovidov, S., Wu, H., Bae, H., Nakajima, K., Kaji, H. & Khademhosseini, A. Engineered nanomembranes for directing cellular organization towards flexible biodevices. Nano Lett. 13, 3185–3192 (2013).
Bettinger, C. J., Langer, R. & Borenstein, J. T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 48, 5406–5415 (2009).
Hosseini, V., Ahadian, S., Ostrovidov, S., Camci-Unal, G., Chen, S., Kaji, H., Ramalingam, M. & Khademhosseini, A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng. Part A 18, 2453–2465 (2012).
Günther, A., Yasotharan, S., Vagaon, A., Lochovsky, C., Pinto, S., Yang, J., Lau, C., Voigtlaender-Bolz, J. & Bolz, S.-S. A microfluidic platform for probing small artery structure and function. Lab Chip 10, 2341–2349 (2010).
Yuan, B., Jin, Y., Sun, Y., Wang, D., Sun, J., Wang, Z., Zhang, W. & Jiang, X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 24, 890–896 (2012).
Shi, X., Fujie, T., Saito, A., Takeoka, S., Hou, Y., Shu, Y., Chen, M., Wu, H. & Khademhosseini, A. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv. Mater. 26, 3290–3296 (2014).
Fujie, T., Shi, X., Ostrovidov, S., Liang, X., Nakajima, K., Chen, Y., Wu, H. & Khademhosseini, A. Spatial coordination of cell orientation directed by unique nanoribbon sheets. Biomaterials 53, 86–94 (2015).
Hynes, S. R. & Lavik, E. B. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes. Arch. Clin. Exp. Ophthalmol. 248, 763–778 (2010).
Binder, S., Stanzel, B. V., Krebs, I. & Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 26, 516–554 (2007).
Fujie, T., Mori, Y., Ito, S., Nishizawa, M., Bae, H., Nagai, N., Onami, H., Abe, T., Khademhosseini, A. & Kaji, H. Micropatterned polymeric nanosheets for local delivery of an engineered epithelial monolayer. Adv. Mater. 26, 1699–1705 (2014).
Fukuda, K., Takeda, Y., Yoshimura, Y., Shiwaku, R., Tran, L. T., Sekine, T., Mizukami, M., Kumaki, D. & Tokito, S. Fully-printed high-performance organic thin-film transistors and circuitry on one-micron-thick polymer films. Nat. Commun. 5, 4147 (2014).
Dagdeviren, C., Shi, Y., Joe, P., Ghaffari, R., Balooch, G., Usgaonkar, K., Gur, O., Tran, P. L., Crosby, J. R., Meyer, M., Su, Y., Chad Webb, R., Tedesco, A. S., Slepian, M. J., Huang, Y. & Rogers, J. A. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 14, 728–736 (2015).
Rivnay, J., Owens, R. M. & Malliaras, G.G. The rise of organic bioelectronics. Chem. Mater. 26, 679–685 (2014).
Zucca, A., Yamagishi, K., Fujie, T., Takeoka, S., Mattoli, V. & Greco, F. Roll to roll processing of ultraconformable conducting polymer nanosheets. J. Mater. Chem. C 3, 6539–6548 (2015).
Acknowledgements
This work was partially supported by JSPS KAKENHI (grant number 15H05355 for TF) from MEXT, Japan, the Precursory Research for Embryonic Science and Technology (PRESTO) from the Japan Science and Technology Agency (JST; grant number 15655478 for TF), and Mizuho Foundation for the Promotion of Sciences (TF). The author sincerely acknowledge Professor Shinji Takeoka and Mr Kento Yamagishi at Waseda University, Professor Daizoh Saitoh and Associate Professor Manabu Kinoshita at the National Defense Medical College, Associate Professor Yosuke Okamura at Tokai University, Professor Ali Khademhosseini at the Harvard-MIT Division of Health Sciences and Technology, Associate Professor Hirokazu Kaji and Professor Toshiaki Abe at Tohoku University, and Dr Alessandra Zucca, Dr Francesco Greco and Dr Virgilio Mattoli at the Italian Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Fujie, T. Development of free-standing polymer nanosheets for advanced medical and health-care applications. Polym J 48, 773–780 (2016). https://doi.org/10.1038/pj.2016.38
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2016.38
This article is cited by
-
Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds
Scientific Reports (2021)
-
Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy
Nature Biomedical Engineering (2018)
-
Cellular behaviors on polymeric scaffolds with 2D-patterned mechanical properties
Polymer Journal (2018)
-
Renewable polymeric materials for electronic applications
Polymer Journal (2017)
-
On the injectability of free-standing magnetic nanofilms
Biomedical Microdevices (2017)