Abstract
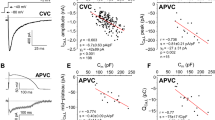
ABSTRACT: Action potentials and voltage clamp-induced ionic currents were recorded in acutely isolated neonatal rabbit cardiac myocytes using the whole-cell voltage clamp technique. Time- and voltage-dependent Ca2+ currents in neonatal myocytes were elicited by depolarizations from a holding potential of —80 mV to various clamp potentials. The maximal measured inward Ca2+ current was 206 ± 10 pA (mean ± SEM, n = 51). The peak current occurred at a mean membrane potential of 7.8 ± 1.3 mV (n = 51). The Ca2+ current voltage relation was shifted 26 mV in the positive direction when the external Ca2+ concentration was increased 10-fold. Ca2+ current rundown was observed with a half-time of approximately 20 min. Cells dialyzed with solution containing the Ca2+ chelating agent, EGTA (0.04 mM), had action potential durations similar to those previously reported in papillary muscle. In contrast, a higher concentration of EGTA (14 mM) prolonged the action potential duration. Control of the cell internal ionic composition was achieved by dialysis of the cell with a time constant for Na+ ions of 1.2 to 2.6 min. Tetrodotoxin (10 μM), included in some experiments to block Ca2+ entry via Na+ channels, was shown to be more than 98% effective. These results characterize the whole-cell voltage clamp technique as applied to immature heart cells.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wetzel, G., Chen, F., Friedman, W. et al. Calcium Current Measurements in Acutely Isolated Neonatal Cardiac Myocytes. Pediatr Res 30, 83–88 (1991). https://doi.org/10.1203/00006450-199107000-00017
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199107000-00017