Abstract
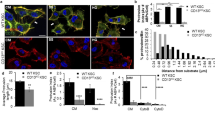
The newborn has a limited ability to regulate H+/HCO-3 homeostasis, due in part to immaturity of the intercalated cells in the distal nephron. We traced the postnatal differentiation of the intercalated cells of the rabbit cortical collecting duct (CCD) and outer medullary collecting duct(OMCD) using MAb to the 31-kD subunit of the vacuolar H+-ATPase, membrane portion of erythrocyte band 3, and apical surface of B-intercalated cells (peanut agglutinin [PNA], MAb B63). In the most superficial CCD of the newborn there was no binding to these probes, although deeper in the cortex there was faint apical staining with PNA and MAb B63 and a few patterns of H+-ATPase and band 3 labeling of neonatal intercalated cells. The OMCD showed mostly apical H+-ATPase and both cytoplasmic and basolateral band 3 labeling but B-intercalated cell markers were not seen. By 3 wk of age the staining of the CCD and OMCD was more polarized, resembling those in the adult. Band 3 positive cells (as a percentage of total cells) in the CCD increased from 13 to 17% during maturation, and in the OMCD they increased from 22 to 37%. Some basolateral band 3 and apical H+-ATPase staining was also seen in the inner medullary collecting duct of 3-wk-old rabbits to a greater extent than in newborn or adult rabbits. Labeling of intercalated cells in the CCD and OMCD was weakest and least numerous in the newborn, greater in the 3 wk old, and greatest in the adult. Most maturing cortical intercalated cells bound both PNA and H+-ATPase MAb, comparable to what has been observed in the adult CCD. PNA-negative cells showing apical H+-ATPase labeling, consistent with the classic A-intercalated cell phenotype, comprised only 5% of identified intercalated cells in the newborn CCD compared with 12% in older animals. In or near the developing renal vesicles and ampullary structures were occasional cytoplasmically staining PNA- and B63-positive cells. Whether these cells are precursors of specific renal tubular cells cannot yet be established. Staining for principal cells(ST.9) was less intense in the neonatal cortex than in more mature cortex, but the deep cortex and outer medulla were heavily labeled at all ages. These data indicate that immature intercalated cells, in the CCD and OMCD, may undergo significant postnatal proliferation and differentiation, acquiring mature phenotypes during the first month of life. The A-intercalated cell appears more differentiated than the B cell during the 1st wk of life, suggesting that A-intercalated cells contribute more than B cells to the maintenance of acid-base homeostasis in the newborn.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CCD:
-
cortical collecting duct
- OMCD:
-
outer medullary collecting duct
- IMCD:
-
inner medullary collecting duct
- PNA:
-
peanut agglutinin
References
Stork EK, Stork JE 1987 Acid-base physiology and disorders in the neonate. In: Faranoff AA Martin RJ (eds) Neonatal-Perinatal Medicine: Diseases of the Fetus and Infant, 4th Ed. CV Mosby, St. Louis, 466–472
Spitzer A, Schwartz GJ 1992 The kidney during development. In: Windhager EE (ed) Handbook of Physiology, Section 8: Renal Physiology. Oxford University Press, New York, 475–544
Edelmann CM Jr, Rodriguez-Soriano J, Biochis H, Gruskin AB, Acosta M 1967 Renal bicarbonate reabsorption and hydrogen ion excretion in infants. J Clin Invest 46: 1309–1317
Edelmann CM Jr, Spitzer A 1969 The maturing kidney: a modern view of well-balanced infants with imbalanced nephrons. J Pediatr 75: 509–519
Baum M 1990 Neonatal rabbit juxtamedullary proximal convoluted tubule acidification. J Clin Invest 85: 499–506
Mehrgut FM, Satlin LM, Schwartz GJ 1990 Maturation of HCO-3 transport in rabbit collecting duct. Am J Physiol 259:F801–F808
Moore ES, Fine BP, Satrasook SS, Vergel ZM, Edelmann CM Jr 1972 Renal absorption of bicarbonate in puppies: effect of extracellular volume concentration on the renal threshold for bicarbonate. Pediatr Res 6: 859–867
Schwartz GJ, Evan AP 1983 Development of solute transport in rabbit proximal tubule. I. HCO-3 and glucose absorption. Am J Physiol 245:F382–F390
Alpern RJ, Stone DK, Rector FC Jr 1991 Renal acidification mechanisms. In: Brenner BM, Rector FC Jr (eds) The Kidney, 4th Ed. WB Saunders, Philadelphia, pp 318–379
Koeppen BM, Giebisch G, Malnic G 1985 Mechanism and regulation of renal tubular acidification. In: Seldin DW, Giebisch G (eds) The Kidney. Physiology and Pathophysiology. Raven, New York, pp 1491
Madsen KM, Tisher CC 1986 Structural-functional relationships along the distal nephron. Am J Physiol 250:F1–F15
Schwartz GJ, Barasch J, Al-Awqati Q 1985 Plasticity of functional epithelial polarity. Nature 318: 368–371
Ridderstrale Y, Kashgarian M, Koeppen B, Giebisch G, Stetson D, Ardito T, Stanton B 1988 Morphological heterogeneity of the rabbit collecting duct. Kidney Int 34: 655–670
Satlin LM, Matsumoto T, Schwartz GJ 1992 Postnatal maturation of rabbit renal collecting duct. III. Peanut lectin-binding intercalated cells. Am J Physiol 262:F199–F208
Weiner ID, Hamm LL 1989 Use of fluorescent dye BCECF to measure intracellular pH in cortical collecting tubule. Am J Physiol 256:F957–F964
Fejes-Toth G, Naray-Fejes-Toth A, Satlin LM, Mehrgut FM, Schwartz GJ 1994 Inhibition of bicarbonate transport in peanut lectin-positive intercalated cells by a monoclonal antibody. Am J Physiol 266:F901–F910
Schuster VL, Fejes-Toth G, Naray-Fejes-Toth A, Gluck S 1991 Colocalization of H+ ATPase and band 3 anion exchanger in rabbit collecting duct intercalated cells. Am J Physiol 260:F506–F517
Lombard WE, Kokko JP, Jacobson HR 1983 Bicarbonate transport in cortical and outer medullary collecting tubules. Am J Physiol 244:F289–F296
McKinney TD, Burg MB 1977 Bicarbonate transport by rabbit cortical collecting tubules. J Clin Invest 60: 766–768
Emmons C, Kurtz I 1994 Functional characterization of three intercalated cell subtypes in the rabbit outer cortical collecting duct. J Clin Invest 93: 417–423
Schwartz GJ, Satlin LM, Bergmann JE 1988 Fluorescent characterization of collecting duct cells: a second H+-secreting type. Am J Physiol 255:F1003–F1014
Graber ML, Bengele HH, Schwartz JH, Alexander EA 1981 pH and PCO2 profiles of the rat inner medullary collecting duct. Am J Physiol 241:F659–F668
Verlander JW, Madsen KM, Stone DK, Tisher CC 1994 Ultrastructural localization of H+ ATPase in rabbit cortical collecting duct. J Am Soc Nephrol 4: 1546–1557
Schuster VL, Bonsib SM, Jennings ML 1986 Two types of collecting duct mitochondria-rich (intercalated) cells: lectin and band 3 cytochemistry. Am J Physiol 251:C347–C355
Brown D, Hirsch S, Gluck S 1988 Localization of a proton-pumping ATPase in rat kidney. J Clin Invest 82: 2114–2126
Clapp WL, Madsen KM, Verlander JW 1987 Intercalated cells of the rat inner medullary collecting duct. Kidney Int 31: 1080–1087
Satlin LM, Schwartz GJ 1987 Postnatal maturation of the rabbit renal collecting duct: intercalated cell function. Am J Physiol 253:F622–F635
Evan AP, Satlin LM, Gattone VH, Connors B, Schwartz GJ 1991 Postnatal maturation of rabbit renal collecting duct. II. Morphological observations. Am J Physiol 261:F91–F107
Narbaitz R, Vandorpe D, Levine DZ 1991 Differentiation of renal intercalated cells in fetal and postnatal rats. Anat Embryol 183: 353–361
Narbaitz R, Kapal VK, Levine DZ 1993 Induction of intercalated cell changes in rat pups from acid- and alkali-loaded mothers. Am J Physiol 264:F415–F420
Matsumoto T, Schwartz GJ 1992 Novel method for performing carbonic anhydrase histochemistry and immunocytochemistry on cryosections. J Histochem Cytochem 40: 1223–1227
Hemken P, Guo X-L, Wang Z-Q, Khang K, Gluck S 1992 Immunologic evidence that vacuolar H+ ATPases with heterogeneous forms of Mr = 31,000 subunit have different membrane distributions in mammalian kidney. J Biol Chem 267: 9948–9957
Peranen J, Kaariainen L, Rikkonen M 1991 Immunofluorescence localization of intracellular proteins after denaturation-applied to initially non-reactive antibodies. J Cell Biol 115: 81a
Fejes-Toth G, Naray-Fejes-Toth A 1987 Differentiated transport functions in primary cultures of rabbit collecting ducts. Am J Physiol 253:F1302–F1307
Brown D, Hirsch S, Gluck S 1988 An H+-ATPase in opposite plasma membrane domains in kidney epithelial cell subpopulations. Nature 331: 622–624
Bastani B, Purcell H, Hemken P, Trigg D, Gluck S 1991 Expression and distribution of renal vacuolar proton-translocating adenosine triphosphatase in response to chronic acid and alkali loads in the rat. J Clin Invest 88: 126–136
Minuth WW, Rudolph U 1990 Successive lectin-binding changes within the collecting duct during post-natal development of the rabbit kidney. Pediatr Nephrol 4: 505–509
Alper SL, Natale J, Gluck S, Lodish HF, Brown D 1989 Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc Natl Acad Sci USA 86: 5429–5433
Weiner ID, Hamm LL 1991 Regulation of Cl-/HCO-3 exchange in the rabbit cortical collecting tubule. J Clin Invest 87: 1553–1558
Weiner ID, Weill AE, New AR 1994 Distribution of Cl-/HCO-3 exchange and intercalated cells in rabbit cortical collecting duct. Am J Physiol 267:F952–F964
Holthofer H 1987 Ontogeny of cell type-specific enzyme reactivities in kidney collecting ducts. Pediatr Res 22: 504–508
Kim J, Tisher CC, Madsen KM 1994 Differentiation of intercalated cells in developing rat kidney: an immunohistochemical study. Am J Physiol 266:F977–F990
Madsen KM, Kim J, Tisher CC 1992 Intracellular band 3 immunostaining in type A intercalated cells of rabbit kidney. Am J Physiol 262:F1015–F1022
Matsumoto T, Winkler CA, Brion LP, Schwartz GJ 1994 Expression of acid-base-related proteins in mesonephric kidney of the rabbit. Am J Physiol 267:F987–F997
Clark SL Jr, 1957 Cellular differentiation in the kidneys of newborn mice studied with the electron microscope. J Biophys Biochem Cytol 3: 349–362
Minuth WW, Gilbert P, Rudolph U, Spielman WS 1989 Successive histochemical differentiation steps during postnatal development of the collecting duct in rabbit kidney. Histochemistry 93: 19–25
Fejes-Toth G, Naray-Fejes-Toth A 1992 Differentiation of renal -intercalated cells to -intercalated and principal cells in culture. Proc Natl Acad Sci USA 89: 5487–5491
Fejes-Toth G, Naray-Fejes-Toth A 1991 Cell differentiation and stem cell renewal via asymmetric cell division in the renal collecting duct. J Cell Biol 115: 148
Satlin LM, Schwartz GJ 1989 Cellular remodeling of HCO-3-secreting cells in rabbit renal collecting duct in response to an acidic environment. J Cell Biol 109: 1279–1288
Minuth WW, Gross P, Gilbert P, Kashgarian M 1987 Expression of the -subunit of Na/K-ATPase in renal collecting duct epithelium during development. Kidney Int 31: 1104–1112
Acknowledgements
The authors are grateful to Dr. Stephen Gluck for providing the MAb E11 to the 31-kD subunit of the vacuolar H+-ATPase and to Dr. Victor Schuster for the MAb IVF12 to the membrane domain of erythrocyte band 3.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grants HD13232 to G.J.S. and DK39523 and DK45647 to G.F.-T.
Parts of this material were presented at the Fifth International Workshop on Developmental Nephrology, Tremezzo, Como, Italy, 26–28 August 1992.
Rights and permissions
About this article
Cite this article
Matsumoto, T., Fejes-Toth, G. & Schwartz, G. Postnatal Differentiation of Rabbit Collecting Duct Intercalated Cells. Pediatr Res 39, 1–12 (1996). https://doi.org/10.1203/00006450-199601000-00001
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199601000-00001
This article is cited by
-
Adaptation to metabolic acidosis and its recovery are associated with changes in anion exchanger distribution and expression in the cortical collecting duct
Kidney International (2010)
-
Replication of segment-specific and intercalated cells in the mouse renal collecting system
Histochemistry and Cell Biology (2007)