Abstract
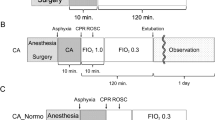
The study was peformed to assess the utility of the Morris water maze (MWM) and acoustic startle reflex (ASR) for evaluating neurologic outcome in a rat model of asphyxial cardiac arrest. Rats were anesthetized, intubated, and chemically paralyzed. Control animals were decannulated and, after awakening, were extubated and returned to their housing. Experimental animals were asphyxiated by disconnecting the ventilator. Approximately 3.5 min after the disconnection, there was no measurable pulse. After 7 min of asphyxia, they were then resuscitated with resumed ventilation, chest compressions, epinephrine, and sodium bicarbonate. All animals were assigned to either MWM or ASR testing. The MWM is a 6-ft diameter tank filled with opaque water. In a fixed location of the tank, a 4-inch diameter escape platform is submerged just below the surface. MWM animals were tested on post-injury d 16-21 by recording the path and time taken to escape from three randomly assigned locations per d. ASR animals had s.c. leads placed over the right triceps and tibialis anterior muscles. The latency and rectified amplitude of the ASR was measured by recording the electromyographic impulse generated when the animal was startled by an acoustic stimulus. Animals were tested on post-injury d 6 and 7. After the last test session for each group, the animals' brains were removed for histopathologic examination. Asphyxiated MWM animals took longer to find the platform, and their paths were less direct than control animals(analysis of variance p < 0.05). The ASR of asphyxiated ASR animals had greater amplitude and shorter latency compared with controls(analysis of variance p < 0.05). Histologic examination revealed no abnormalities in control animals, but 80% of asphyxiated brains showed hippocampal neuronal injury and/or reactive gliosis in the CA1 segment. Abnormalities were more commonly detected in animals killed 7 d post-injury(ASR protocol) compared with animals killed 21 d post-injury (MWM protocol). We conclude that the MWM and ASR are useful for detecting neuronal injury in asphyxiated rats.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MWM:
-
Morris water maze
- ASR:
-
acoustic startle reflex
- H&E:
-
hematoxylin-eosin
- GFAP:
-
glial fibrillary acidic protein
- ANOVA:
-
analysis of variance
References
Kaasik AE, Nilsson L, Siesjo BK 1970 The effect of asphyxia upon the lactate, pyruvate and bicarbonate concentrations of brain tissue and cisternal CSF, and upon the tissue concentrations of phosphocreatine and adenine nucleotides in anesthetized rats. Acta Physiol Scand 78: 433–447
Hendrickx HH, Rao GR, Safar P, Grisvold SE 1984 Asphyxia, cardiac arrest and resuscitation in rats: short term recovery. Resuscitation 12: 97–116
Hendrickx HH, Safar P, Miller A 1984 Asphyxia, cardiac arrest and resuscitation in rats: long term behavioral changes. Resuscitation 12: 117–128
Hendrickx HH, Safar P, Baer BP, Basford RE 1984 Brain lactate and alanineglutamate ratios during and after asphyxia in rats. Resuscitation 12: 129–140
Thiringer K, Hrbek A, Karlsson K, Rosen KG, Kjellmer I 1987 Post-asphyxial cerebral survival in newborn sheep after treatment with oxygen free radical scavengers and a calcium antagonist. Pediatr Res 22: 62–66
Vaagenes P, Safar P, Moossy J, Rao G, Diven W, Cantadore R 1988 Differences in the effects of CNS treatments after ventricular fibrillation (VF) vs. asphyxiation (A) cardiac arrest (CA) in dog models. Crit Care Med 16: 447( abstr)
Safar P 1985 Long-term animal outcome models for cardiopulmonary-cerebral resuscitation research. Crit Care Med 13: 936–940
Kochanek PM 1988 Novel pharmacologic approaches to brain resuscitation after cardiorespiratory arrest in the pediatric patient. Crit Care Clin 4: 661–677
Lindner KH, Ahnefeld FW, Bowdler IM 1990 Cardiopulmonary resuscitation with interposed abdominal compression after asphyxial or fibrillatory cardiac arrest in pigs. Anesthesiology 72: 675–681
Eisenberg M, Bergner L, Hallstrom A 1983 Epidemiology of cardiac arrest and resuscitation in children. Ann Emerg Med 12: 672–674
Safranek DJ, Eisenberg MS, Larsen MP 1992 The epidemiology of cardiac arrest in young adults. Ann Emerg Med 21: 1102–1106
Hickey RW, Cohen DM, Strausbaugh S, Dietrich A 1995 Pediatric patients requiring CPR in the prehospital setting. Ann Emerg Med 25: 495–501
Brandeis R, Brandys Y, Yehuda S 1989 The use of the morris water maze in the study of memory and learning. Int J Neurosci 48: 29–69
Leitner DS, Powers AS, Hoffman HS 1980 The neural substrate of the startle response. Physiol Behav 25: 291–297
Davis M 1989 Neural systems involved in fear-potentiated startle. Ann NY Acad Sci 563: 165–183
Ison JR, McAdam DW, Hammond GR 1973 Latency and amplitude changes in the acoustic startle reflex of the rat produced by variation in auditory pre-stimulation. Physiol Behav 10: 1035–1039
Leitner DS, Powers AS, Stitt CL, Hoffman HS 1981 Midbrain reticular formation involvement in the inhibition of acoustic startle. Physiol Behav 26: 259–268
Hoffman HS, Ison JR 1980 Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 87: 175–189
Morris R 1984 Development of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11: 47–60
Morris RG, Garrud P, Rawlins JNP, O'Keefe J 1982 Place navigation impaired in rats with hippocampal lesions. Nature 297: 681–683
Weisfeldt ML, Halperin HR 1986 Cardiopulmonary resuscitation: beyond cardiac massage. Circulation 74: 443–448
Myers RE, Yamaguchi S 1977 Nervous system effects of cardiac arrest in monkeys. Arch Neurol 34: 65–74
Katz L, Sim KM, Radovsky A, Neumar R, Ubmeyer U, Safar P 1992 Asphyxial cardiac arrest survival model in rats with quantitative brain histopathologic evaluation. Ann Emerg Med 21: 633( abstr)
Vogel FS 1979 The morphologic consequences of cerebral hypoxia. Adv Neurol 26: 147–154
Petito CK, Feldmann E, Pulsinelli WA, Plum F 1987 Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology 37: 1281–1286
Bengtsson M, Holmberg S, Jansson B 1969 A psychiatric-psychological investigation of patients who had survived circulatory arrest. Acta Psychiatr Scand 45: 327–346
Bergner L, Hallstrom AP, Bergner M, Eisenberg MS, Cobb LA 1985 Health status of survivors of cardiac arrest and myocardial infarction controls. Am J Public Health 75: 1321–1323
Zola-Morgan S, Squire LR, Amaral DG 1986 Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci 6: 2950–2967
Volpe BT, Holtzman JD, Hirst W 1986 Further characterization of patients with amnesia after cardiac arrest: preserved recognition of memory. Neurology 36: 408–411
Bertini G, Giglioli C, Giovannini F, Bartoletti A, Cricelli F, Margheri M, Russo L, Taddei T, Taiti A 1990 Neuropsychological outcome of survivors of out-of-hospital cardiac arrest. J Emerg Med 8: 407–412
Roine RO, Kajaste S, Kaste M 1993 Neuropsychological sequelae of cardiac arrest. JAMA 269: 237–242
Morris RD, Krawiecki NS, Wright JA, Walter LW 1993 Neuropsychological, academic, and adaptive functioning in children who survive in-hospital cardiac arrest and resuscitation. J Learn Disabil 26: 46–51
Thompson FJ, Parmer R, Fessler RG 1993 Is spinal cord injury induced frequency-hyperreflexia related to intraspinal GABAb receptor dysfunction? Eleventh Annual Neurotrauma Symposium (abstr)
Miller JR, Myers RE 1972 Neuropathology of systemic circulatory arrest in adult monkeys. Neurology 22: 888–904
de Courten-Myers GM, Fogelson HM, Kleinholz M, et al 1989 Hypoxic brain and heart injury thresholds in piglets. Biomed Biochim Acta 48:S143–S148
Kochanek PM 1993 Ischemic and traumatic brain injury: pathobiology and cellular mechanisms. Crit Care Med 21:S333–S335
Siesjo BK 1988 Mechanisms of ischemic brain damage. Crit Care Med 16: 954–963
de Courten-Myers G, Kleinholz M, Wagner KR, Myers RE 1991 Asphyxia- compared to cardiac arrest-induced brain damage is more extensive and uniquely involves thalamus and brainstem. Neurology 41: 337( abstrt)
Morimoto Y, Kemmotsu O, Kitami K, Matsubara I, Tedo I 1993 Acute brain swelling after out-of-hospital cardiac arrest: pathogenesis and outcome. Crit Care Med 21: 104–110
Katz L, Ebmeyer U, Safar P, Radovsky A, Neumar R 1995 J Cereb Blood Flow Metab (in press)
Acknowledgements
The authors are grateful to Dr. Jean Powers and John Hayes for their help with statistical analysis, and Drs. Bradford Stokes and Charles Brown for their review of the manuscript. We are especially indebted to Drs. Larry Katz and Robert Neumar for their help and advice with the asphyxial model of cardiac arrest in rats.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hickey, R., Akino, M., Strausbaugh, S. et al. Use of the Morris Water Maze and Acoustic Startle Chamber to Evaluate Neurologic Injury after Asphyxial Arrest in Rats. Pediatr Res 39, 77–84 (1996). https://doi.org/10.1203/00006450-199601000-00011
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199601000-00011
This article is cited by
-
Brain Resuscitation in the Drowning Victim
Neurocritical Care (2012)