Abstract
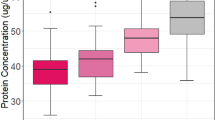
We used two independent in vitro assays to measure the frequency and proliferative potential of primitive hematopoietic progenitors from the cord blood of 23-41 wk of gestation newborns and adult bone marrow. The frequency of primitive progenitors in the circulating blood cells of infants at 23-31 wk of gestation was significantly greater than the frequency in adult bone marrow or cord blood of more mature newborns. In addition, on a cell to cell basis, the proliferative potential of the primitive progenitors from immature infants (23-31 wk) was greater than in adult bone marrow or cord blood of term newborns. Circulating cord blood cells from immature infants were used as targets for transduction with recombinant retrovirus vectors, and a high efficiency of gene transfer was observed in both primitive and committed progenitors. These data demonstrate that there are major ontogenic shifts in primitive progenitor/stem cell populations in the circulation throughout development as well as programmatic changes in hematopoietic progenitor cell proliferation. In addition, fetal cord blood cells may prove useful targets for genetic manipulation and autologous transplantation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- LDMNC:
-
low density mononuclear cells
- IMDM:
-
Iscove's modified Dulbecco's media
- CFU:
-
colony forming unit
- GM:
-
granulocyte/macrophage
- BFU-E:
-
burst forming unit-erythroid
- GEMM:
-
granulocyte/erythroid/macrophage/megakaryocyte
- HPP-CFC:
-
high proliferating potential-colony forming cells
- LTC-IC:
-
long-term culture-initiating cell
- SCID:
-
severe combined immunodeficiency
- rh:
-
recombinant human
References
Simpson JL, Carson SA 1992 Preimplantation genetic diagnosis. N Engl J Med 327: 951–953.
The NICHD National Registry for Amniocentesis Study Group 1976 Midtrimester amniocentesis for prenatal diagnosis: safety and accuracy. JAMA 236: 1471–1476.
Jackson LG, Zachary JM, Fowler SE, Desnick RJ, Golbus MS, Ledbetter DH, Mahoney MJ, Pergament E, Simpson JL, Black S, Wapner RJ, and the U.S. National Institute of Child Health and Human Development Chorionic-Villus Sampling and Amniocentesis Study Group 1992 N Engl J Med 327: 594–598.
MRC Working Party on the Evaluation of Chorion Villus Sampling 1991 Medical research council European trial of chorion villus sampling. Lancet 337: 1491–1499.
Shulman LP, Simpson JL, Elias S, Felker RE, Emerson DS, Phillips OP 1993 Transvaginal chorionic villus sampling using transabdominal ultrasound guidance: a new technique for first-trimester prenatal diagnosis. Fetal Diagn Ther 8: 144–148.
Touraine JL, Raudrant D, Royo C, Rebaud A, Barbier F, Roncarolo MG, Touraine F, Laplace S, Gebuhrer L, Betuel H, Frappaz D, Freycon F, Vullo C 1991 In utero transplantaton of hemopoietic stem cells in humans.. Transplant Proc 23: 1706–1708.
Blazar BR, Taylor PA, Vallera DA 1995 Adult bone marrow-derived pluripotent hematopoietic stem cells are engraftable when transferred in utero into moderately anemic fetal recipients. Blood 85: 833–841.
Flake AW, Hedrick MH, Rice HE, Tavassoli M, Zanjani ED 1995 Enhancement of human hematopoiesis by mast cell growth factor in human-sheep chimeras created by the in utero transplantation of human fetal hematopoietic cells. Exp Hematol 23: 252–257.
Zanjani ED, Almeida-Porada G, Flake AW 1995 Retention and multilineage expression of human hematopoietic stem cells in human-sheep chimeras. Stem Cells 13: 101–111.
Metcalf D, Moore MAS 1971 Haematopoietic cells. In Neuberger A, Tatum EL (eds) Frontiers of Biology. North Holland, Amsterdam, pp 448–465.
Moore MAS, Metcalf D 1970 Ontogeny of the hematopoietic system: Yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol 18: 279–286.
Migliaccio G, Migliaccio AR, Petti S, Mavilio F, Russo G, Lazzaro D, Testa U, Marinucci M, Peschle E 1986 Human embryonic hemopoiesis-kinetics of progenitors and precursors underlying the yolk sac liver transition. J Clin Invest 78: 51
Fleischman RA, Custer RP, Mintz B 1982 Totipotent hematopoietic stem cells: normal self-renewal and differentiation after transplantation between mouse fetuses. Cell 30: 3351–3359.
Jordan CT, McKearn JP, Lemischka IR 1990 Cellular and developmental properties of fetal hematopoietic stem cells. Cell 61: 953–963.
Clapp DW, Freie B, Lee W, Zhang Y 1995 Molecular evidence that in situ transduced fetal liver hematopoietic stem/progenitor cells give rise to medullary hematopoiesis in adult rats. Blood 86: 2113–2122.
Clapp DW, Baley JE, Gerson SL 1989 Gestational age dependent changes in circulating hematopoietic stem cells in newborn infants. J Lab Clin Med 113: 422–427.
Lu L, Xiao M, Clapp DW, Lu ZH, Broxmeyer HE 1993 High efficiency retroviral-mediated gene transduction into single isolated immature and replatable CD34+++ hematopoietic stem/progenitor cells from human umbilical cord blood. J Exp Med 178: 1089–1096.
Freie B, Dutt P, Clapp, DW Correction of Fanconi anemia type C phenotypic abnormalities using a clinically-suitable retroviral vector infection protocol. Cell Transplant ( in press)
Broxmeyer HE, Douglas DW, Hangoc G, Cooper S, Bard J, English D, Arny M, Thomas L, Boyse EA 1989 Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA 86: 3828–3832.
Moritz T, Keller DC, Williams DA 1993 Human cord blood as targets for gene transfer. Potential use in genetic therapies of severe combined immunodeficiency disease. J Exp Med 78: 519–536.
Lu L, Xiao M, Shen RN, Grigsby S, Broxmeyer HE 1993 Enrichment, characterization and responsiveness of single primitive CD34+++ human umbilical cord blood hematopoietic progenitors with high proliferative and replating potential. Blood 81: 41–48.
Sutherland HJ, Lansdorp P, Henkelman DH, Eaves AC, Eaves CJ 1990 Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci USA 87: 3584–3588.
Clapp DW, Freie B, Srour E, Yoder MC, Fortney K, Gerson SL 1995 Myeloproliferative sarcoma virus directed expression of-galactosidase following retroviral transduction of murine hematopoietic cells. Exp Hematol 23: 630–638.
Lu L, Ge Y, Li Z-H, Freie B, Clapp DW, Broxmeyer HE 1995 CD34+++ stem/progenitor cells purified from cryopreserved normal cord blood can be transduced with high efficiency by a retroviral vector and expanded ex vivo with stable integration and expression of Fanconi anemia complementation C gene. Cell Transplant 4: 1–15.
McNiece IK, Stewart FM, Deacon DM, Temeles DS, Zsebo KM, Clark SC, Quesenaberry PJ 1989 Detection of human CFC with a high proliferative potential. Blood 74: 609–612.
Srour EF, Zanjani ED, Brandt JE, Leembuis T, Briddell RA, Heerema NA, Hoffman R 1992 Sustained human hematopoiesis in sheep transplanted in utero during early gestation with fractionated adult human bone marrow cells. Blood 79: 1404–1412.
Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE 1992 Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science 255: 1137–1141.
Vormoor J, Lapidot T, Pflumio F, Risdon G, Patterson B, Broxmeyer HE, Dick JE 1994 Immature human cord blood progenitors engraft and proliferate to high levels in immune-deficient SCID mice. Blood 83: 2489–2497.
Christensen RD, Harper TE, Rothstein G 1986 Granulocyte-macrophage progenitor cells in term and preterm neonates. J Pediatr 109: 1047–1051.
Linch DC, Knott LG, Rodeck CH, Huehns ER 1983 Studies of circulating hematopoietic progenitor cells in human fetal blood. Blood 59: 976–979.
Fleischman RA, Custer RP, Mintz B 1982 Totipotent hematopoietic stem cells: Normal self-renewal and differentiation after transplantation between mouse fetuses. Cell 30: 351–359.
Blanchet JP, Fleischman RA, Mintz B 1982 Murine adult hematopoietic cells produce adult erythrocytes in fetal recipients. Dev Genet 3: 197–203.
Harrison DE, Astle CM, Lerner C 1984 Ultimate erythropoietic repopulating abilities of fetal, young adult, and old adult cells compared using repeated irradiation. J Exp Med 160: 759
Lansdorp PM, Dragowska W, Mayani H 1993 Ontogeny-related changes in proliferative potential of human hematopoietic cells. J Exp Med 178: 787–791.
Clapp DW, Williams DA 1995 The use of umbilical cord blood as a cellular source for correction of genetic diseases affecting the hematopoietic system. Stem Cells 13: 613–621.
Kohn DB, Weinberg KI, Parkman R, Lenarsky C, Crooks GM, Shaw K, Hanley ME, Lawrence K, Annett G, Brooks JS, Wara D, Elder M, Bowen T, Hershfield MS, Berenson RI, Moen RC, Muller CA, Blaese M 1994 Gene therapy for neonates with ADA-deficient SCID by retroviral-mediated transfer of the human ADA cDNA into umbilical cord CD34+ cells. Blood 82: 315a
Acknowledgements
The authors thank Lisa Weber and Nancy Muessig at St. Vincent's Hospital in Indianapolis, IN, as well as Dr. Dawn Zimmer in the Department of Obstetrics and Gynecology at Indiana University and Dr. Lyle Ahuja at Saint Francis Hospital for assistance in obtaining blood specimens used in these studies. We also thank Dr. Li Lu of the Walther Oncology Center at Indiana University for blindly scoring representative cord blood specimens. We thank Drs. Li Lu, Hal Broxmeyer, David Williams, and Mervin Yoder for the numerous helpful discussions over the course of these experiments. In addition, we thank Pat Fox for secretarial assistance in preparing this manuscript.
Author information
Authors and Affiliations
Additional information
The Wells Center for Pediatric Research is a Center of Excellence in Molecular Hematology funded by the National Institute of Diabetes and Digestive and Kidney Diseases (P50DK49218) and the Riley Memorial Association. D.W.C. is the recipient of a Clinical Investigator Award National Heart, Lung, and Blood Institute (K08 HL02721) and a Basil O'Connor Award from the March of Dimes Birth Defects Foundation (#5-FY94-0820). This work is also funded in part by the Indiana affiliate of the American Heart Association.
Rights and permissions
About this article
Cite this article
Haneline, L., Marshall, K. & Clapp, D. The Highest Concentration of Primitive Hematopoietic Progenitor Cells in Cord Blood Is Found in Extremely Premature Infants. Pediatr Res 39, 820–825 (1996). https://doi.org/10.1203/00006450-199605000-00013
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199605000-00013
This article is cited by
-
Placental transfusion: may the “force” be with the baby
Journal of Perinatology (2021)
-
Effect of delayed cord clamping on stem cell transfusion and hematological parameters in preterm infants with placental insufficiency: a pilot randomized trial
European Journal of Pediatrics (2021)
-
Circulating hematopoietic stem cell count is a valuable predictor of prematurity complications in preterm newborns
BMC Pediatrics (2012)
-
Effects of delayed cord clamping in very-low-birth-weight infants
Journal of Perinatology (2011)