Abstract
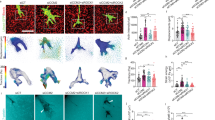
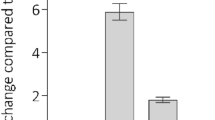
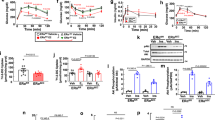
Anatomical closure of the ductus arteriosus requires normally quiescent luminal endothelial cells and medial smooth muscle cells to migrate into the subendothelial space forming intimal mounds that eventually coalesce and occlude the vessel's lumen. The migration of endothelial cells and smooth muscle cells requires the presence of integrin receptors that interact with the surrounding matrix. We used immunohistochemical staining to examine the repertoires of integrins expressed by endothelial cells and smooth muscle cells during postnatal closure of the ductus arteriosus in full-term and preterm rhesus monkeys. In the fetal ductus, luminal endothelial cells have a limited repertoire of integrins. During postnatal ductus closure, luminal endothelial cells, of both term and preterm monkeys, change their phenotype and express the full repertoire of integrins found on growing capillary endothelial cells (α1β1,α2β1, α3β1,α6β1, αvβ1,α6β4, and αvβ5). Similarly, during ductus closure, smooth muscle cells of both term and preterm monkeys expand their integrin repertoire to include the α5β1 and αvβ3 integrins; these two integrins have been shown to be essential for smooth muscle cell migration in vitro. These changes in integrin profile occur at the same time the endothelial and smooth muscle cells invade their neighboring compartments. In contrast, preterm monkeys with a persistently patent ductus lumen fail to develop these changes in integrin expression and fail to develop neointimal mounds. No evidence of intimal thickening occurs in the absence of changes in integrin expression. Therefore, endothelial cells and smooth muscle cells change phenotypes to produce the intimal thickening required for ductus closure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- EC:
-
endothelial cell(s)
- SMC:
-
smooth muscle cell(s)
- Fio2:
-
fractional concentration of inspired oxygen
- PECAM:
-
platelet EC adhesion molecule
References
Slomp J, van Munsteren JC, Poelmann RE, de Reeder EG, Bogers AJ, Gittenberger-de Groot AC 1992 Formation of intimal cushions in the ductus arteriosus as a model for vascular intimal thickening: an immunohistochemical study of changes in extracellular matrix components. Atherosclerosis 93: 25–39
de Reeder EG, Girard N, Poelmann RE, Van Munsteren JC, Patterson DF, Gittenberger-de-Groot AC 1988 Hyaluronic acid accumulation and endothelial cell detachment in intimal thickening of the vessel wall: the normal and genetically defective ductus arteriosus. Am J Pathol 132: 574–585
Tannenbaum JE, Waleh NS, Mauray F, Gold L, Perkett EA, Clyman RI Transforming growth factor-β protein and messenger RNA expression is increased in the closing ductus arteriosus. Pediatr Res 39: 427–434
Gittenberger-de-Groot AC, Strengers JLM, Mentink M, Poelmann RE, Patterson DF 1985 Histologic studies on normal and persistent ductus arteriosus in the dog. J Am Coll Cardiol 6: 394–404
de Reeder EG, van Munsteren CJ, Poelmann RE, Patterson DF, Gittenberger-de-Groot AC 1990 Changes in distribution of elastin and elastin receptor during intimal cushion formation in the ductus arteriosus. Anat Embryol 182: 473–480
Yoder MJ, Baumann FG, Grover-Johnson NM, Brick I, Imparato AM 1978 A morphological study of early cellular changes in the closure of the rabbit ductus arteriosus. Anat Rec 192: 19–39
de Reeder EG, Poelmann RE, van Munsteren JC, Patterson DF, Gittenberger-de-Groot AC 1989 Ultrastructural and immunohistochemical changes of the extracellular matrix during intimal cushion formation in the ductus arteriosus of the dog. Atherosclerosis 79: 29–40
Gittenberger-de-Groot AC, Van Ertbruggen I, Moulaert AJMG, Harinck E 1980 The ductus arteriosus in the preterm infant: histologic and clinical observations. J Pediatr 96: 88–93
Ruoslahti E 1991 Integrins. J Clin Invest 87: 1–5
Hynes RO 1992 Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25
Damjanovich L, Albelda SM, Mette SA, Buck CA 1992 Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am J Respir Cell Mol Biol 6: 197–206
Enenstein J, Kramer RH 1994 Confocal microscopic analysis of integrin expression on the microvasculature and its sprouts in the neonatal foreskin. J Invest Dermatol 103: 381–386
Korhonen M, Ylanne J, Laitinen L, Virtanen I 1990 Theα1-α6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol 111: 1245–1254
Defilippi P, van Hinsbergh V, Bertolotto A, Rossino P, Silengo L, Tarone G 1991 Differential distribution and modulation of expression of α1/β1 integrin on human endothelial cells. J Cell Biol 114: 855–863
Brooks PC, Clark RA, Cheresh DA 1994 Requirement of vascular integrin αvβ3 for angiogenesis. Science 264: 569–571
Roussel E, Gingras MC, Ro JY, Branch C, Roth JA 1994 Loss of α1 β1 and reduced expression of other β1 integrins and CAM in lung adenocarcinoma compared with pneumocytes. J Surg Oncol 56: 198–208
Mechtersheimer G, Barth T, Hartschuh W, Lehnert T, Moller P 1994 In situ expression of β1, β3 and β4 integrin subunits in non-neoplastic endothelium and vascular tumours, Virchows A. rchiv 425: 375–384
Pasqualini R, Bodorova J, Ye S, Hemler ME 1993 A study of the structure, function and distribution of β5 integrins using novel anti-β5 monoclonal antibodies. J Cell Sci 105: 101–111
Rudolph R, Cheresh D 1990 Cell adhesion mechanisms and their potential impact on wound healing and tumor control. Clin Plast Surg 17: 457–462
Skinner MP, Raines EW, Ross R 1994 Dynamic expression ofα1β1 and α2β1 integrin receptors by human vascular smooth muscle cells: α2β1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol 145: 1070–1081
Glukhova M, Koteliansky V, Fondacci C, Marotte F, Rappaport L 1993 Laminin variants and integrin laminin receptors in developing and adult human smooth muscle. Dev Biol 157: 437–447
Schnapp LM, Breuss JM, Ramos DM, Sheppard D, Pytela R 1995 Sequence and tissue distribution of the human integrin α8 subunit: a β1-associated α subunit expressed in smooth muscle cells. J Cell Sci 108: 537–544
Palmer EL, Rüegg C, Ferrando R, Pytela R, Sheppard D 1993 Sequence and tissue distribution of the integrin α9 subunit, a novel partner of β1 that is widely distributed in epithelia and muscle. J Cell Biol 123: 1289–1297
Goetzman BW, Read LC, Plopper CG, Tarantal AF, George-Nascimento C, Merritt TA, Whitsett JA, Styne D 1994 Prenatal exposure to epidermal growth factor attenuates respiratory distress syndrome in rhesus infants. Pediatr Res 35: 30–36
Deng JS, Beutner EH 1974 Effect of formaldehyde, glutaraldehyde and sucrose on the tissue antigenicity. Int Arch Allergy Appl Immunol 47: 562–569
Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL 1984 Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol 132: 3011–3018
Morhenn VB, Roth S, Roth R 1987 Use of a monoclonal antibody (VM-2) plus the immunogold-silver technique to stain basal cell carcinoma cells. J Am Acad Dermatol 17: 765–769
Wayner EA, Carter WG 1987 Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique α and common β subunits. J Cell Biol 105: 1873–1884
Sonnenberg A, Hogervost F, Osterop A, Veltman FE 1988 Identification and characterization of a novel antigen complex on mouse mammary tumor cells using a monoclonal antibody against platelet glycoprotein Ic. J Biol Chem 263: 14030–14038
Weinacker A, Chen A, Agrez M, Cone RI, Nishimura S, Wayner E, Pytela R, Sheppard D 1994 Role of the integrinαvβ6 in cell attachment to fibronectin: heterologous expression of intact and secreted forms of the receptor. J Biol Chem 269: 6940–6948
Cheresh DA, Spiro RC 1987 Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem 262: 17703–17711
Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH 1989 Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109: 877–889
Albelda SM, Oliver PD, Romer LH, Buck CA 1990 EndoCAM: a novel endothelial cell-cell adhesion molecule. J Cell Biol 110: 1227–1237
Rosa JP, McEver RP 1989 Processing and assembly of the integrin, glycoprotein IIb-IIIa, in HEL cells. J Biol Chem 264: 12596–12603
Heino J, Ignotz RA, Hemler ME, Crouse C, Massague J 1989 Regulation of cell adhesion receptors by transforming growth factor-β: concomitant regulation of integrins that share a common β1 subunit. J Biol Chem 264: 380–388
Zhu L, Dagher E, Johnson DJ, Bedell-Hogan D, Keeley FW, Kagan HM, Rabinovitch M 1993 A developmentally regulated program restricting insolubilization of elastin and formation of laminae in the fetal lamb ductus arteriosus. Lab Invest 68: 321–331
Clarke JA 1965 An x-ray microscopic study of the vasa vasorum of the human ductus arteriosus. J Anat 99: 527–537
Clyman RI, Mauray F, Kramer RH 1992 β1 andβ3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res 200: 272–284
Schwartz MA, Denninghoff K 1994 Alpha v integrins mediate the rise in intracellular calcium in endothelial cells on fibronectin even though they play a minor role in adhesion. J Biol Chem 269: 11133–11137
Pasqualini R, Hemler ME 1994 Contrasting roles for integrin β1 and β5 cytoplasmic domains in subcellular localization, cell proliferation, and cell migration. J Cell Biol 125: 447–460
Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband JL 1994 Specific roles of the αvβ1,αvβ3 and αvβ5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development 120: 2687–2702
Clyman RI, Tannenbaum J, Chen YQ, Cooper D, Yurchenco PD, Kramer RH, Waleh NS 1994 Ductus arteriosus smooth muscle cell migration on collagen: dependence on laminin and its receptors. J Cell Sci 107: 1007–1018
Boudreau N, Turley E, Rabinovitch M 1991 Fibronectin, hyaluron and a hyaluron binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol 143: 235–247
Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J 1993 Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest 92: 1425–1435
Clark RAE 1990 Fibronectin matrix deposition and fibronectin receptor expression in healing and normal skin. J Invest Dermatol 94 ( suppl 6): 128S–134S
Juhasz I, Murphy GF, Yan HC, Herlyn M, Albelda SM 1993 Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healingin vivo. Am J Pathol 143: 1458–1469
Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA 1993 Integrin β1- and β3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol 121: 163–170
Blystone SD, Graham IL, Lindberg FP, Brown EJ 1994 Integrin αvβ3 differentially regulates adhesive and phagocytic functions of the fibronectin receptorα5β1 . J Cell Biol 127: 1129–1137
Weiss H, Cooper B, Brook M, Schlueter M, Clyman RI 1995 Factors determining reopening of the ductus arteriosus after successful clinical closure with indomethacin. J Pediatr 127: 466–471
Acknowledgements
The authors thank Paul Sagan for his expert editorial assistance, and Dr. T. Allen Merritt for assisting us in obtaining monkey tissue.
Author information
Authors and Affiliations
Additional information
Supported in part by U.S. Public Health Service, National Heart, Lung, Blood Institute Grants HL46691, HL56061, and HD24959, a grant from the W. H. Tooley Memorial Fund, and a gift from the Perinatal Associates Research Foundation.
Rights and permissions
About this article
Cite this article
Clyman, R., Goetzman, B., Chen, Y. et al. Changes in Endothelial Cell and Smooth Muscle Cell Integrin Expression during Closure of the Ductus Arteriosus: An Immunohistochemical Comparison of the Fetal, Preterm Newborn, and Full-Term Newborn Rhesus Monkey Ductus. Pediatr Res 40, 198–208 (1996). https://doi.org/10.1203/00006450-199608000-00004
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199608000-00004