Abstract
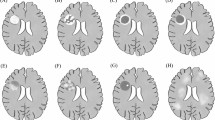
Lymphoproliferative disorders (LPDs) are commoner in pediatric versus adult immunosuppressed transplant recipients, and frequently involve the central nervous system. In these circumstances, the justification for biopsy is heavily influenced by the likely diagnostic yield. The present study centers on a 12-y-old renal transplant patient who developed multifocal cerebral LPD and had serial magnetic resonance (MR) examinations during the course of her illness from which she has completely recovered upon reduction of immunosuppression. She underwent stereotaxic biopsy, which was analyzed by both immunocytochemistry and polymerase chain reaction to examine the general question of how to release the maximum amount of information contained within, as well as to obtain a tissue diagnosis in this particular case. We show that a combination of these methods permits identification of the immunophenotype, lineage, clonality, viral involvement, and origin of abnormal cellular infiltrates. The biopsy also showed a novel histologic pattern of LPD, comprising numerous benign T cells obscuring a tiny clone of B cells. The MR examinations documented, for the first time, the differences in signal that accompany clinical resolution at both biopsied and nonbiopsied sites, showing that the latter may be associated with reduction, but not elimination, of MR signal abnormality. We conclude: 1) a combination of conventional and polymerase chain reaction analysis offers the greatest diagnostic yield from stereotaxic biopsies, even when the available tissue is minimal;2) a focal polyclonal T cell infiltrate should prompt further investigation to exclude an underlying B cell lesion.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- LPD:
-
lymphoproliferative disorder
- MR:
-
magnetic resonance
- CMV:
-
cytomegalovirus
- PCR:
-
polymerase chain reaction
- VCA:
-
viral capsid antigen
- IgH:
-
immunuglobulin heavy-chain
- TCR:
-
T cell receptor
- HUMARA:
-
human androgen receptor
References
Nalesnik MA, Jaffe R, Starzl TE, Demetris AJ, Porter K, Burnham JA, Makowka L, Ho M, Locker J 1988 The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine-prednisolone immunosuppression. Am J Pathol 133: 173–192
Swerdlow SH 1992 Post-transplant lymphoproliferative disorders: a morphologic, phenotypic and genotypic spectrum. Histopathology 20: 373–385
Fischer A, Blanche S, Le Bidois J, Bordigoni P, Garnier PL, Niaudet P, Morinet F, Le Deist F, Fischer A-M, Grischelli G, Hirn M 1991 Anti-B-cell monoclonal antibodies in the treatment of severe B-cell lymphoproliferative syndrome following bone marrow and organ transplantation. N Engl J Med 324: 1451–1456
Penn I, Porat G 1995 Central nervous system lymphomas in organ allograft recipients. Transplantation 59: 240–244
Wright DK, Manos MM 1990 Sample preparation from paraffin-embedded tissues. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, eds. PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego, pp 153–158
Diss TC, Huaizheng P, Wotherspoon AC, Langxing P, Speight PM, Isaacson PG 1993 Brief report: a single neoplastic clone in sequential biopsy specimens from a patient with primary gastric-mucosa-associated lymphoid-tissue lymphoma and Sjogren's syndrome. N Engl J Med 329: 172–175
McCarthy KP, Sloane JP, Kabarowski JHS, Matutes E, Wiedemann LM 1992 A simplified method of detection of clonal rearrangements of the T-cell receptor-γ chain gene. Diagn Mol Pathol 1: 173–179
Diss TC, Watts M, Pan LX, Burke M, Linch D, Isaacson PG 1995 The polymerase chain reaction in the demonstration of monoclonality in T cell lymphomas. J Clin Pathol 48: 1045–1050
Brocksmith D, Angel CA, Pringle JH, Lauder I 1991 Epstein-Barr viral DNA in Hodgkin's disease: amplification and detection using the polymerase chain reaction. J Pathol 165: 11–15
Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW 1992 Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor correlates with X chromosome inactivation. Am J Hum Genet 51: 1229–1239
Ho M, Jaffe R, Miller G, Breinig MK, Dummer SJ, Makowka L, Atchison RW, Karrer F, Nalesnik MA, Starzl TE 1988 The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation 45: 719–727
McKhann CF 1969 Primary malignancy in patients undergoing immunosuppression for renal transplantation. Transplantation 8: 209–212
Penn I, Hammond W, Brettschneider L, Starzl TE 1969 Malignant lymphomas in transplantation patients. Transplant Proc 1: 106–112
Hanto DW, Frizzera G, Gajl-Peczalska KJ, Simmons RL 1985 Epstein-Barr virus, immunodeficiency and B-cell lymphoproliferation. Transplantation 39: 461–472
Ulrich W, Chott A, Watschinger B, Reiter C, Kovarik J 1989 Primary peripheral T-cell lymphoma in a kidney transplant under immunosupression with cyclosporin A. Hum Pathol 20: 1027–1030
Cleary ML, Warnke R, Sklar J 1984 Monoclonality of lymphoproliferative lesions in cardiac-transplant recipients. N Engl J Med 310: 477–482
Sumaya CV 1977 Primary Epstein-Barr virus infection in children. Pediatrics 59: 16–21
Ho M, Miller G, Atchison RW, Breinig MK, Dummer JS, Andiman W, Starzl TE, Eastman R, Griffith BP, Hardesty RL, Bahnson HT, Hakala TR, Rosenthal JT 1985 Epstein-Barr virus infections and DNA hybridization studies in posttransplant lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis 152: 876–886
Osterhage D, Steele PE, Witte D, Schroeder T, Yochman L, Swerdlow SH 1992 T-cells in posttransplant lymphoproliferative disorders: an immunophenotypic and genotypic investigation. Lab Invest 66: 85A
Ferry JA, Jacobson JO, Conti D, Delmonico F, Harris NL 1989 Lymphoproliferative disorders and hematological malignancies following organ transplantation. Mod Pathol 2: 583–592
Canioni D, MacKelvie P, Debure A, Nezelof C 1989 Lymphadenopathy in renal transplant patients treated with immunosuppressive antibodies (OKT3 and anti-thymocyte globulin). A report of nine cases. Am J Surg Pathol 13: 87–96
Thomas JA, Hotchin NA, Allday MJ, Amlot P, Rose M, Yacoub M, Crawford DH 1990 Immunohistology of Epstein-Barr virus-associated antigens in B-cell disorders from immunocompromised individuals. Transplantation 49: 944–953
Hjelle B, Evans-Holm B, Yen TSB, Garavoy M, Guis M, Edman JC 1989 A poorly-differentiated lymphoma of donor origin in a renal allograft recipient. Transplantation 47: 945–948
Meduri G, Fromentin L, Viellefond A, Fries D 1991 Donor-related non-Hodgkin's lymphoma in a renal allograft recipient. Transplant Proc 23: 2649
Spiro IJ, Yandell DW, Chuan L, Saini S, Ferry F, Powelson J, Katkov WN, Cosimi AB 1993 Brief report: lymphoma of donor origin occurring in the porta hepatis of a transplanted liver. N Engl J Med 329: 27–29
Tubman DE, Frick MP, Hanto DW 1983 Lymphoma after organ transplantation: radiologic manifestations in the central nervous system, thorax and abdomen. Radiology 149: 625–631
Acknowledgements
The authors thank Dr. Huai Zheng Peng for providing the protocol for amplification of the HUMARA locus, Dr. Rosemary Gale for assistance in its interpretation, and Dr. Anthea Tilzey for discussion of the serologic data, and to Alan Brady for photography.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dean, A., Diss, T., Wotherspoon, A. et al. Histologic, Molecular, and Radiologic Characterization of Resolving Cerebral Posttransplant Lymphoproliferative Disorder. Pediatr Res 41, 651–656 (1997). https://doi.org/10.1203/00006450-199705000-00009
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199705000-00009
This article is cited by
-
Neurologic complications of organ transplantation
Current Treatment Options in Neurology (2001)