Abstract
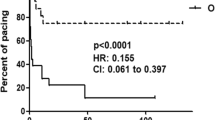
Although a strong clinical association exists between congenital heart block (CHB) and an immune response to SSA/Ro and SSB/La proteins, a causative role of these antibodies in the pathogenesis is just emerging. In a preliminary report, we have demonstrated that IgG fractions isolated from the sera of mothers whose children have CHB are arrhythmogenic in the human fetal heart. To more precisely define the arrhythmogenic effect of anti-SSA/Ro-SSB/La antibodies, we used the readily available rat heart model to record: 1) ECGs from Langendorff beating hearts;2) action potentials from atrioventricular (AV) nodal preparations;3) L-type Ca currents, ICa at the whole-cell and single channel levels; and 4) other currents such as the transient outward K+ current,Ito, the inward rectifier K+ current,IK1, and the Na+ current,INa. Perfusion of hearts with purified IgG (800 µg/mL), isolated from the serum of a mother with SSA/Ro and SSB/La antibodies whose child had CHB, resulted in bradycardia associated with 2:1 AV block. Simultaneous action potentials were recorded from dissected atrial and AV nodal areas of the rat heart. Superfusion of these preparations with the same mother's IgG fraction resulted in 2:1 AV block followed by complete inhibition of AV nodal action potential. Because AV nodal electrogenesis is largely dependent on ICa, the effect of these antibodies on ICa was subsequently determined. Superfusion of myocytes with whole serum or purified IgG (80 µg/mL) from the same mother consistently inhibited whole cell ICa, ensemble average Ba2+ currents (IBa) and open state probability, po, without affecting the channel conductance. IgG had no significant effect on Ito,IK1, or INa. Whole sera and IgG fractions from a healthy mother with no detectable anti-SSA/Ro or SSB/La antibodies did not inhibit ICa or IBa. These results demonstrate that IgG containing anti-SSA/Ro and -SSB/La antibodies induces complete AV block in beating hearts and in multicellular preparations, thus implicating a preferential interaction of these autoantibodies with Ca channels and/or associated regulatory proteins. This is consistent with the observed inhibition of Ca channels that may be a critical factor contributing to the pathogenesis of CHB.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AV :
-
atrioventricular
- CHB :
-
congenital heart block
- I Na :
-
sodium current
- I Ca :
-
L-type Ca current
- I to :
-
transient outward K current
- I K1 :
-
inward rectifier K current
References
Waltuck J, Buyon J 1994 Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Int Med 120: 544–551.
Buyon JP, Winchester RJ 1990 Complete congenital heart block: a model of passively acquired autoimmunity. Arthritis Rheum 33: 609–614.
Buyon JP 1992 Neonatal lupus syndromes. In: Lahita R (ed) Systemic Lupus Erythematosus. Churchill Livingstone, New York, 419–435.
Lee LA 1990 Maternal autoantibodies and pregnancy. II. The neonatal lupus syndrome. In: Parke AL (ed) Bailliere's Clinical Rheumatology, Pregnancy and the Rheumatic Diseases. WB Saunders, Philadelphia 69–84.
Copel J, Buyon J, Kleinman C 1995 Successful in utero therapy of fetal heart block. Am J Obstet Gynecol 173: 1384–1390.
Buyon JP, Ben-Chetrit E, Karp S, Roubey RAS, Pompeo L, Reeves WH, Tan EM, Winchester R 1989 Acquired congenital heart block: pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J Clin Invest 84: 627–634.
Silverman ED, Mamula MJ, Hardin JA, Laxer RM 1991 The importance of the immune response to the Ro/La particle in the development of complete heart block and neonatal lupus erythematosus. J Rheumatol 18: 120–124.
Lee LA, Frank MB, McCubbin VR, Reichlin M 1994 The autoantibodies of neonatal lupus erythematosus. J Invest Dermatol 102: 963–966.
Julkunen H, Siren MK, Kaaja R, Kurki P, Friman C, Koskimies S 1995 Maternal HLA antigens and antibodies to SS-A/Ro and SS-B/La. Comparison with systemic lupus erythematosus and primary Sjogren's syndrome. Br J Rheumatol 34: 901–907.
Brucato A, Franceschini F, Gasparini M, De Juli E, Ferraro G, Quinzanini M, Vignati G, Bortolon C, Ghessi A, Possoli R 1995 Isolated congenital complete heart block: longterm outcome of mothers, maternal antibody specificity and immunogenetic background. J Rheumatol 22: 533–540.
Deutscher SL, Harley JB, Keene JD 1988 Molecular analysis of the 60 kD human Ro ribonucleoprotein. Proc Natl Acad Sci USA 85: 9479–9483.
Ben-Chetrit E, Gandy BJ, Tan EM, Sullivan KF 1989 Isolation and characterization of a cDNA clone encoding the 60-kD component of the human SS-A/Ro ribonucleoprotein autoantigen. J Clin Invest 83: 1284–1292.
Lerner MR, Boyle JA, Hardin JA, Steitz JA 1981 Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science 211: 400–402.
O'Brien CA, Wolin SL 1994 A possible role for the 60 kD Ro autoantigen in a discard pathway for defective 5s rRNA precursors. Genes Dev 8: 2891–2903.
Chan EKL, Sullivan KF, Tan EM 1989 Ribonucleoprotein SS-B/La belongs to a protein family with consensus sequences for RNA-binding. Nucleic Acids Res 17: 2233–2244.
Chambers JC, Kenan D, Martin BJ, Keene JD 1988 Genomic structure and amino acid sequence domains of the human La autoantigen. J Biol Chem 263: 18043–18051.
Gottlieb E, Steitz JA 1989 Function of the mammalian La protein: evidence for its action in transcription termination by RNA polymerase III. EMBO J 8: 851–861.
Boire B, Craft J 1990 Human Ro ribonucleoprotein particles: characterization of native structure and stable association with the La polypeptide. J Clin Inv 85: 1182–1190.
Ben-Chetrit E, Chan EKL, Sullivan KF, Tan EM 1988 A 52 kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med 162: 1560–1571.
Chan EKL, Hamel JC, Buyon JP, Tan EM 1991 Molecular definition and sequence motifs of the 52-kD component of human SS-A/Ro autoantigen. J Clin Invest 87: 68–76.
Itoh K, Itoh Y, Frank MB 1991 Protein heterogeneity in the human Ro/SSA ribonucleoproteins. J Clin Invest 87: 177–186.
Garcia S, Nascimento JHM, Bonfa E, Levy R, Oliveira SF, Tavares AV, Campos deCarvalho AC 1994 Cellular mechanism of the conduction abnormalities induced by serum from anti-Ro/SSA-positive patients in rabbit hearts. J Clin Invest 93: 718–724.
Boutjdir M, Chen L, Zhang Z, Tseng C, DiDonato F, Rashbaum W, Morris A, El-Sherif N, Buyon JP 1997 Arrhythmogenicity of IgG and anti-52 kD SSA/Ro affinity purified antibodies from mothers of children with congenital heart block. Circ Res 80: 354–362.
Paes de Carvalho A, De Mello W, Hoffman BF 1959 Electrophysiological evidence for specialized fiber types in rabbit atrium. Am J Physiol 196: 483–488.
Le Heuzey JY, Guize L, Valty J, Moutet JP, Kouz S, Lavergne T, Boutjdir M, Peronneau P 1986 Intracellular and extracellular recordings of sinus node activity: comparison with estimated sinoatrial conduction times during pacemaker shifts in rabbit heart. Cardiovasc Res 20: 81–88.
Boutjdir M, El Sherif N 1991 α1-Adrenoceptor regulation of delayed afterdepolarizations and triggered activity in subendocardial Purkinje fibers surviving 1 day of myocardial infarction. J Mol Cell Cardiol 23: 83–90.
Hancox J, Levi AJ, Lee CO, Heap P 1993 A method for isolating rabbit atrioventricular node myocytes which retain normal morphology and function. Am J Physiol 265:H755–H766.
Chen L, El-Sherif N, Boutjdir M 1996 α1-Adrenergic activation inhibits Beta-adrenergic stimulated unitary Ca current in cardiac ventricular myocytes. Circ Res 79: 184–193.
Zhang ZH, Johnson JA, El-Sherif N, Mochly-Rosen D, Boutjdir M 1997 C2 region-derived peptides of protein kinase C regulates cardiac Ca2+ channels. Circ Res 80: 720–729.
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ 1981 Improved patch-clamp techniques for high-resolution current recording from cell and cell-free membrane patches. Pflugers Arch 391: 85–100.
Zhang ZH, Boutjdir M, El-Sherif N 1994 Ketanserin inhibits depolarization-activated outward potassium current in rat ventricular myocytes. Circ Res 75: 711–721.
Scamps F, Legssyer A, Mayoux E, Vassort G 1990 The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res 67: 1007–1016.
Zipes DP, Mendez C 1973 Action of manganese ions and tetrodotoxin on atrioventricular nodal transmembrane potentials in isolated rabbit hearts. Circ Res 32: 447–454.
Hancox J, Levi AJ 1994 L-type calcium current in rod- and spindle-shaped myocytes isolated from rabbit atrioventricular node. Am J Physiol 267:H1670–H1680.
Geggel RL, Tucker L, Szer I 1988 Postnatal progression from second- to third-degree heart block in neonatal lupus syndrome. J Pediatr 113: 1049–1052.
Bacman S, Sterin-Borda L, Camusso JJ, Hubscher O, Arana R, Sterin-Borda E 1994 Circulating antibodies against neurotransmitter receptor activities in children with congenital heart block and their mothers. FASEB J 8: 1170–1176.
Herreman G, Galezewski N 1985 Maternal connective tissue disease and congenital heart block [Letter]. N Engl J Med 312: 1329
Lee LA, Coulter S, Erner S, Chu H 1987 Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro antibody. Am J Med 83: 793–796.
Litsey SE, Noonan JA, O'Conner WN, Cottrill CM, Mitchell B 1985 Maternal connective tissue disease and congenital heart block. N Engl J Med 312: 98–100.
Ho YS, Esscher E, Anderson RH, Michaelsson M 1986 Anatomy of congenital complete heart block and relation to maternal anti-Ro antibodies. Am J Cardiol 58: 291–294.
Delbono O, Garcia J, Appel SH, Stefani E 1991 Calcium current and charge movement of mammalian muscle action of amyotrophic lateral sclerosis immunoglobulins. J Physiol 444: 723–742.
Acknowledgements
The authors thank the animal laboratory staff at the Veterans Administration Medical Center for their technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by National Heart, Lung and Blood Institute Grant HL-55401(M.B.), Veterans Administration Medical Research Funds (M.B.), Grant AR-42455 from the National Institute of Arthritis, Musculoskeletal, and Skin Diseases(J.P.B.), and a grant-in-aid from the American Heart Association, New York Affiliate (J.P.B.).
Rights and permissions
About this article
Cite this article
Boutjdir, M., Chen, L., Zhang, ZH. et al. Serum and Immunoglobulin G from the Mother of a Child with Congenital Heart Block Induce Conduction Abnormalities and Inhibit L-Type Calcium Channels in a Rat Heart Model. Pediatr Res 44, 11–19 (1998). https://doi.org/10.1203/00006450-199807000-00002
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199807000-00002
This article is cited by
-
Autoimmune channelopathies as a novel mechanism in cardiac arrhythmias
Nature Reviews Cardiology (2017)
-
Neonatal congenital heart block
Indian Pediatrics (2013)
-
Congenital heart block: evidence for a pathogenic role of maternal autoantibodies
Arthritis Research & Therapy (2012)
-
Pregnancy Outcomes in Patients with Autoimmune Diseases and Anti-Ro/SSA Antibodies
Clinical Reviews in Allergy & Immunology (2011)
-
Congenital heart block in neonatal lupus: The pediatric cardiologist’s perspective
The Indian Journal of Pediatrics (2002)