Abstract
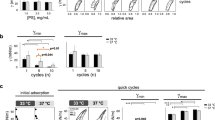
We studied the mechanisms by which C16:0 lysophosphatidylcholine (LPC) and albumin inhibit the surface activity of calf lung surfactant extract (CLSE) by using a pulsating bubble apparatus with a specialized hypophase exchange system, plus adsorption and Wilhelmy balance measurements. In the absence of inhibitors, CLSE (1 mg phospholipid/mL) reached minimum surface tension (γmin) < 1 mN/m within 5 min of bubble pulsation at 20 cycles/min at 37°C. Mixtures of CLSE:LPC had impaired surface activity depending on LPC content: γmin was raised to 5 mN/m by 14 wt % LPC, to 15 mN/m by 25-30 wt % LPC, and to >20 mN/m (67 wt % LPC), even at high CLSE concentrations (3 and 6 mg phospholipid/mL). In contrast, inhibition of CLSE by albumin was more easily abolished when surfactant concentration was raised. Mixtures of albumin (3 mg/mL) and CLSE (1 mg phospholipid/mL) had γmin >20 mN/m, but normal values of γmin < 1 mN/m were reached at higher CLSE concentration (3 mg phospholipid/mL) even when albumin concentration was increased 8-fold to 24 mg/mL. In hypophase exchange studies, LPC, but not albumin, was able to penetrate preformed CLSE surface films and raise γmin. CLSE surface films with γmin < 1 mN/m were isolated by an initial hypophase exchange with saline, and a second exchange with an LPC- containing hypophase raised γmin to ∼10 mN/m. CLSE surface films retained the ability to reach γmin < 1 mN/m in analogous hypophase exchange studies with albumin. The ability of LPC to penetrate surface films of CLSE, although albumin could not, was also demonstrated in absorption experiments in a Teflon dish, where diffusion was minimized by subphase stirring. Wilhelmy balance experiments also demonstrated that LPC could mix and interact with CLSE or dipalmitoyl phosphatidylcholine in solvent-spread surface films. The ability of LPC or other cell membrane lipids to penetrate interfacial films and raise γmin even at high surfactant concentration may increase their inhibitory actions during acute lung injury.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- ARDS:
-
acute respiratory distress syndrome
- BAL:
-
bronchoalveolar lavage
- BSA:
-
bovine serum albumin
- CLSE:
-
calf lung surfactant extract
- DPPC:
-
1,2-dipalmitoyl-sn-3-phosphocholine dipalmitoyl phosphatidylcholine
- HEPES:
-
HEPES standard buffer
- LPC:
-
C16:0 lysophosphatidylcholine
- γmin:
-
minimum surface tension
References
Ashbaugh D, Bigelow D, Petty T, Levine B 1967 Acute respiratory distress in adults. Lancet 2: 319–323
Petty TL, Ashbaugh DG 1971 The adult respiratory distress syndrome: clinical features, factors influencing prognosis and principles of management. Chest 60: 233–239
Lewis JF, Jobe AH 1993 Surfactant and ARDS. Am Rev Respir Dis 147: 218–233
Seeger W, Gunther A, Walmrath HD, Grimminger F, Lasch HG 1993 Alveolar surfactant and adult respiratory distress syndrome: pathogenic role and therapeutic prospects. Clin Invest 71: 177–190
Bernard GR, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R, and the Consensus Committee 1994 The American-European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149: 818–824
Koleff MH, Schuster DP 1995 The acute respiratory distress syndrome. N Engl J Med 332: 27–37
Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, Hammerle AF, Steltzer H 1996 The acute respiratory distress syndrome; definitions, severity and clinical outcome: an analysis of 101 clinical investigations. Intensive Care Med 22: 519–529
Notter RH, Wang Z 1997 Pulmonary surfactant: physical chemistry, physiology, and replacement. Rev Chem Eng 13: 1–118
Holm BA, Notter RH, Finkelstein JN 1985 Surface property changes from interactions of albumin with natural lung surfactant and extracted lung lipids. Chem Phys Lipids 38: 287–298
Seeger W, Stöhr G, Wolf HRD, Neuhof H 1985 Alteration of surfactant function due to protein leakage: special interaction with fibrin monomer. J Appl Physiol 58: 326–338
Holm BA, Notter RH 1987 Effects of hemoglobin and cell membrane lipids on pulmonary surfactant activity. J Appl Physiol 63: 1434–1442
Fuchimukai T, Fujiwara T, Takahashi A, Enhorning G 1987 Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 62: 429–437
Holm BA, Enhorning G, Notter RH 1988 A biophysical mechanism by which plasma proteins inhibit lung surfactant activity. Chem Phys Lipids 49: 49–55
Keough KMW, Parsons CS, Tweeddale MG 1989 Interactions between plasma proteins and pulmonary surfactant: pulsating bubble studies. Can J Physiol Pharmacol 67: 663–668
Cockshutt AM, Weitz J, Possmayer F 1990 Pulmonary surfactant-associated protein A enhances the surface activity of lipid extract surfactant and reverses inhibition by blood proteins in vitro. Biochemistry 29: 8424–8429
Venkitaraman AR, Hall SB, Whitsett JA, Notter RH 1990 Enhancement of biophysical activity of lung surfactant extracts and phospholipid-apoprotein admixtures by surfactant protein A. Chem Phys Lipids 56: 185–194
Venkitaraman AR, Baatz JE, Whitsett JA, Hall SB, Notter RH 1991 Biophysical inhibition of synthetic phospholipid-surfactant protein admixtures by plasma proteins. Chem Phys Lipids 57: 49–57
Hall SB, Lu RZ, Venkitaraman AR, Hyde RW, Notter RH 1992 Inhibition of pulmonary surfactant by oleic acid: mechanisms and characteristics. J Appl Physiol 72: 1708–1716
Seeger W, Grube C, Gunther A, Schmidt R 1993 Surfactant inhibition by plasma proteins: differential sensitivity of various surfactant preparations. Eur Respir J 6: 971–977
Holm BA, Notter RH, Siegle J, Matalon S 1985 Pulmonary physiological and surfactant changes during injury and recovery from hyperoxia. J Appl Physiol 59: 1402–1409
Berry D, Ikegami M, Jobe A 1986 Respiratory distress and surfactant inhibition following vagotomy in rabbits. J Appl Physiol 61: 1741–1748
Liau DF, Barrett CR, Bell ALL, Ryan SF 1987 Functional abnormalities of lung surfactant in experimental acute alveolar injury in the dog. Am Rev Respir Dis 136: 395–401
Hall SB, Notter RH, Smith RJ, Hyde RW 1990 Altered function of pulmonary surfactant in fatty acid lung injury. J Appl Physiol 69: 1143–1149
Lewis J, Ikegami M, Jobe A 1990 Altered surfactant function and metabolism in rabbits with acute lung injury. J Appl Physiol 69: 2303–2310
Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L 1982 Evidence of lung surfactant abnormality in respiratory failure. J Clin Invest 70: 673–683
Seeger W, Pison U, Buchhorn R, Obestacke U, Joka T 1990 Surfactant abnormalities and adult respiratory failure. Lung ( suppl): 891–902
Gregory T, Longmore W, Moxley M, Whitsett J, Reed C, Fowler A, Hudson L, Maunder R, Crim C, Hyers T 1991 Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 65: 1976–1981
Holm BA, Keicher L, Liu M, Sokolowski J, Enhorning G 1991 Inhibition of pulmonary surfactant function by phospholipases. J Appl Physiol 71: 1–5
Enhorning G, Shumel B, Keicher L, Sokolowski J, Holm BA 1992 Phospholipases introduced into the hypophase affect the surfactant film outlining a bubble. J Appl Physiol 73: 941–945
Notter RH, Finkelstein J, Taubold R 1983 Comparative adsorption of natural lung surfactant, extracted phospholipids, and artificial phospholipid mixtures to the air-water interface. Chem Phys Lipids 33: 67–80
Notter RH, Penney DP, Finkelstein JN, Shapiro D 1986 Adsorption of natural LS and phospholipid extracts related to tubular myelin formation. Pediatr Res 20: 97–101
Bligh E, Dyer W 1959 A rapid method of total lipid extraction and purification. Can J Biochem 37: 911–917
Ames BN 1966 Assay of inorganic phosphate, total phosphate and phosphatases. Meth Enzymol 8: 115–118
Lowry OH, Rosebrough NJ, Farr AL, Randall RH 1951 Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275
Touchstone JC, Chen JC, Beaver KM 1980 Improved separation of phospholipids in thin layer chromatography. Lipids 15: 61–62
Hall SB, Wang Z, Notter RH 1994 Separation of the hydrophobic components of calf lung surfactant. J Lipid Res 35: 1386–1394
Enhorning G . 1977 Pulsating bubble technique for evaluation of pulmonary surfactant. J Appl Physiol 43: 198–203
Hall SB, Bermel MS, Ko YT, Palmer HJ, Enhorning G, Notter RH 1993 Approximations in the measurement of surface tension on the oscillating bubble surfactometer. J Appl Physiol 75: 467–478
Tabak SA, Notter RH 1977 A modified technique for dynamic surface pressure and relaxation measurements at the air-water interface. Rev Sci Instrum 48: 1196–1201
Stafford RE, Fanni T, Dennis EA 1989 Interfacial properties and critical micelle concentrations of lysophospholipids. Biochemistry 28: 5113–5120
Tabak SA, Notter RH 1977 Effects of plasma proteins on the dynamic π-A characteristics of films of saturated phospholipids. J Colloid Interface Sci 59: 293–300
Hall SB, Hyde RW, Notter RH 1994 Changes in subphase aggregates in rabbits injured by free fatty acid. Am J Respir Crit Care Med 149: 1099–1106
Holm BA, Venkitaraman AR, Enhorning G, Notter RH 1990 Biophysical inhibition of synthetic lung surfactants. Chem Phys Lipids 52: 243–250
Amirkhanian JD, Taeusch HW 1993 Reversible and irreversible inactivation of preformed pulmonary surfactant surface films by changes in subphase constituents. Biochim Biophys Acta 1165: 321–326
Helenius A, Simon K 1975 Solubilization of membranes by detergents. Biochim Biophys Acta 415: 27–79
Weltzien HU 1979 Cytolytic and membrane-perturbing properties of lysophosphatidylcholine. Biochim Biophys Acta 559: 259–287
Malik AB 1983 Pulmonary edema after pancreatitis: role of humoral factors. Circ Shock 10: 71–80
Vadas P, Pruzanski W 1986 Role of secretory phospholipase A2 in the pathobiology of disease. Lab Invest 55: 391–404
Vadas P 1984 Elevated plasma phospholipase A2 levels: correlation with the hemodynamic and pulmonary changes in gram-negative shock. J Lab Clin Med 104: 837–881
Offenstadt G, Pinta P, Masliah J, Alcindor LG, Hericord P, Amstutz P 1981 Phospholipase and phospholipasic activities in bronchoalveolar lavage fluid in severe acute pulmonary diseases with or without ARDS. Intensive Care Med 7: 285–291
Von Wichert P, Temmesfeld M, Meyer W 1981 Influence of septic shock upon phosphatidylcholine remodeling mechanisms in rat lung. Biochim Biophys Acta 664: 487–492
Wang Z, Notter RH 1998 Additivity of protein and non-protein inhibitors of lung surfactant. Am J Respir Crit Care Med 158: 28–35
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grant HL-56176.
Rights and permissions
About this article
Cite this article
Holm, B., Wang, Z. & Notter, R. Multiple Mechanisms of Lung Surfactant Inhibition. Pediatr Res 46, 85–93 (1999). https://doi.org/10.1203/00006450-199907000-00015
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199907000-00015
This article is cited by
-
Endogenous lung surfactant inspired pH responsive nanovesicle aerosols: Pulmonary compatible and site-specific drug delivery in lung metastases
Scientific Reports (2014)
-
The surfactant protein C mutation A116D alters cellular processing, stress tolerance, surfactant lipid composition, and immune cell activation
BMC Pulmonary Medicine (2012)
-
A non-BRICHOS surfactant protein c mutation disrupts epithelial cell function and intercellular signaling
BMC Cell Biology (2010)