Abstract
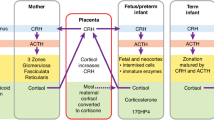
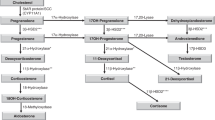
Allopregnanolone is the best characterized among neurosteroids, and its role in the control of neuroendocrine axes has attracted increasing interest recently. However, there is no available information about circulating levels of allopregnanolone during infancy, childhood and puberty. We studied two groups of children: 1) those aged between 0 and 2 y (n = 72), and 2) those aged between 6 and 18 y, at different Tanner's stages (n = 82). In each of these patients, serum allopregnanolone, progesterone, cortisol, and dehydroepiandrosterone levels were evaluated after informed consent; allopregnanolone was measured by RIA after acid extraction on cartridge. There was no significant variation of serum allopregnanolone levels either in male and female children during the first 2 y of life. Furthermore, although serum dehydroepiandrosterone levels showed a significant decrease, inversely correlated with age of the children (p < 0.01), serum cortisol and progesterone levels showed a significant age-related increase during the first 2 y of life. Cortisol and allopregnanolone levels were positively correlated (p < 0.01). During puberty, we observed a progressive increase in serum allopregnanolone levels in both boys and in girls, which were higher at Tanner's stage IV-V (0.7 ± 0.01 nM; mean ± SEM) than at stages I-II (0.32 ± 0.02 nM; p < 0.01); mean levels were significantly higher at puberty than in the first 2 y of life (p < 0.01). Furthermore, during puberty, serum progesterone and dehydroepiandrosterone levels also increased progressively with age in both boys and girls. Allopregnanolone and dehydroepiandrosterone levels were positively correlated throughout puberty. The present results indicate that serum allopregnanolone levels do not change during the first 2 y of life but increase during pubertal development, suggesting that this steroid may be involved in the adaptive neuroendocrine mechanisms related to puberty.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DHEA:
-
dehydroepiandrosterone
- GnRH:
-
gonadotropin releasing hormone
- CRF:
-
corticotropin releasing factor
- GABA:
-
γ-aminobutyric acid
References
Robel P, Baulieu EE 1995 Neurosteroids: biosynthesis and function. Crit Rev Neurobiol 9: 383–394.
Le Goascogne C, Robel P, Gouézou M, Sananés N, Baulieu EE, Waterman M 1987 Neurosteroids: cytochrome P-450scc in rat brain. Science 237: 1212–1215.
Jung-Testas I, Hu ZY, Baulieu EE, Robel P 1989 Steroid synthesis in rat brain cell cultures. J Steroid Biochem 34: 511–519.
Jung-Testas I, Hu ZY, Baulieu EE, Robel P 1989 Neurosteroid: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology 125: 2083–2091.
Majewska MD 1992 Neurosteroids: endogenous bimodal modulators of the GABAa receptor mechanism of action and physiological significance. Prog Neurobiol 38: 379–395.
Paul SM, Purdy RH 1992 Neuroactive steroids. FASEB J 6: 2311–2322.
Mellon SH 1994 Neurosteroids: biochemistry, modes of action, and clinical relevance. J Clin Endocrinol Metab 78: 1003–1008.
Palumbo MA, Salvestroni C, Gallo R, Guo A-L, Genazzani AD, Artini PG, Petraglia F, Genazzani AR 1995 Allopregnanolone concentration in hippocampus of prepubertal rats and female rats throughout estrous cycle. J Endocrinol Invest 18: 853–856.
Genazzani AR, Palumbo MA, de Micheroux AA, Artini PG, Criscuolo M, Ficarra G, Guo A-L, Benelli A, Bertolini A, Petraglia F, Purdy RH 1995 Evidence for a role for the neurosteroid allopregnanolone in the modulation of reproductive function in female rats. Eur J Endocrinol 133: 375–380.
Flood JF, Roberts E 1988 Dehydroepiandrosterone sulfate improves memory in aging mice. Brain Res 447: 269–278.
Wiebe JP, Dhanvantari S, Watson PH, Huang Y 1994 Suppression in gonadotropes of gonadotropin releasing hormone-stimulated follicle-stimulating hormone release by the gonadal- and neurosteroid 3α-hydroxy-4-pregnen-20-one involves cytosolic calcium. Endocrinology 134: 377–382.
Wiebe JP, Wood PH 1987 Selective suppression of follicle-stimulating hormone by 3α-hydroxy-4-pregnen-20-one, a steroid found in Sertoli cells. Endocrinology 120: 2259–2264.
Purdy RH, Moore PH Jr, Rao N, Hagino N, Yamaguchi, Schmidt P, Rubinow DR, Morrow AL, Paul SM 1990 Radioimmunoassay of 3α-hydroxy-5α-pregnan-20-one in rat and in human plasma. Steroids 55: 290–295.
Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Silvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M 1998 Circulating levels of allopregnanolone in humans: gender, age and endocrine influences. J Clin Endocrinol Metab 83: 2099–2103.
Wang M, Seippel L, Purdy RH, Bäckström T 1996 Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5-pregnane 3:20-dione and 3-hydroxy-5-pregnan-20-one. J Clin Endocrinol Metab 81: 1076–1082.
Hopper BR, Yen SC 1975 Circulating concentrations of dehydroepiandrosterone and dehydroepiandrosterone sulfate during puberty. J Clin Endocrinol Metab 40: 458–461.
Freeman EW, Purdy RH, Coutifaris C, Rickels K, Paul SM 1993 Anxiolytic metabolites of progesterone: correlation with mood and performance measures following oral progesterone administration to healthy female volunteers. Neuroendocrinology 58: 478–484.
Caspi A, Moffitt TE 1991 Individual differences are accentuated during periods of social change: the sample case of girls at puberty. J Pers Soc Psychol 61: 157–168.
Xiaojia G, Conger RD, Elder GH 1996 Coming of age too early: pubertal influences on girls' vulnerability to psychological distress. Child Dev 67: 3386–3400.
Grumbach MM, Richards GE, Conte FA, Kaplan SL 1978 Clinical disorders of adrenal function and puberty: an assessment of the role of the adrenal cortex in normal and abnormal puberty in man and evidence for an ACTH-like pituitary adrenal androgen stimulating hormone. In: Serio M (ed) The Endocrine Function of the Human Adrenal Cortex. Serono Symposia, Academic Press, New York, 583–612.
Reiter EO, Fuldauer VG, Root AW 1977 Secretion of the adrenal androgen, dehydroepiandrosterone sulfate, during normal infancy, childhood, and adolescence, in sick infants, and in children with endocrinologic abnormalities. J Pediatr 90: 766–770.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fadalti, M., Petraglia, F., Luisi, S. et al. Changes of Serum Allopregnanolone Levels in the First 2 Years of Life and during Pubertal Development. Pediatr Res 46, 323–327 (1999). https://doi.org/10.1203/00006450-199909000-00013
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199909000-00013
This article is cited by
-
Family environment and development in children adopted from institutionalized care
Pediatric Research (2022)
-
Gender-specific differences in hypothalamus–pituitary–adrenal axis activity during childhood: a systematic review and meta-analysis
Biology of Sex Differences (2017)
-
Uncovering steroidopathy in women with autism: a latent class analysis
Molecular Autism (2014)
-
The role of ovarian hormone-derived neurosteroids on the regulation of GABAA receptors in affective disorders
Psychopharmacology (2014)
-
Differential responses of two related neurosteroids to methylphenidate based on ADHD subtype and the presence of depressive symptomatology
Psychopharmacology (2014)