Abstract
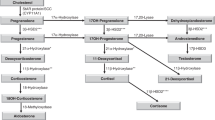
Adrenarche is the increase of adrenal androgen secretion, mainly dehydroepiandrosterone and dehydroepiandrosterone sulfate, that occurs during prepuberty in higher primates. This event takes place at about 6-8 y of age in humans. It had been postulated that adrenarche might reflect an increase in the 17,20 lyase:17OH-ase activity ratio of microsomal cytochrome P450c17. However, studies to demonstrate this mechanism have been unsuccessful. Because it has been described that virilizing adrenocortical carcinomas have high dehydroepiandrosterone sulfate secretion and low 3β-hydroxysteroid dehydrogenase (3βHSD) activity, in this study we evaluated the possible existence of maturative changes of the level of 3βHSD type II mRNA in 11 normal prepubertal and early pubertal human adrenals. Adrenal glands from subjects aged 0.1 to 13 y were obtained from organ donors, patients undergoing resection of the kidney for renal neoplasms or necropsies with less than 6 h of postportem time. The expression of 3βHSD type II gene was studied by dot blot in all samples and by relative reverse transcriptase (RT)-PCR in nine samples. The size of the transcripts was evaluated by Northern blot. Hybridization was performed using labeled human 3βHSD cDNA probes. The uniformity of loading was tested using labeled human βactine cDNA. The relative intensities of hybridization signals were quantified by scanning densitometry. The expected bands after relative RT-PCR were eluted, and radioactivity was measured in a scintillation counter. For the analysis of the results, subjects were divided into two groups as a function of age: group 1, less than 8 y (n = 6; range 0.1-2.48 y) and group 2, equal or older than 8 y (n = 5; range 8-13 y). 3βHSD type II mRNA expression, analyzed by dot blot and relative RT-PCR, was significantly higher (p < 0.05) in group 1 (median and range 4.99, 0.50-8.00 and 16.3, 13.5-40.0 arbitrary units, respectively) than in group 2 (0.15, 0.12-0.75 and 5.66, 3.18-13.0, respectively). In conclusion, we have shown a decrease of the expression 3βHSD type II gene as a function of age in prepubertal and early pubertal normal human adrenal tissue. This maturative change might be involved in the mechanism of human adrenarche.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- DHEA :
-
dehydroepiandrosterone
- P450c17 :
-
cytochrome P450c17
- 3βHSD :
-
3β-hydroxysteroid dehydrogenase
- RT :
-
reverse transcriptase
References
Sklar CA, Kaplan SL, Grumbach MM 1980 Evidence for dissociation between adrenarche and gonadarche: studies in patients with idiopathic precocious puberty, gonadal dysgenesis, isolated gonadotropin deficiency, and constitutionally delayed growth and adolescence. J Clin Endocrinol Metab 51: 548–556.
Parker LN 1991 Adrenarche. Endocrinol Metab Clin North Am 20: 71–78.
Migeon CJ, Keller AR, Laurence B, Shepard THI 1957 Dehydroepiandrosterone and androsterone levels in human plasma: effect of age and sex, day to day and diurnal variations. J Clin Endocrinol Metab 17: 1051–1056.
Miller WL 1988 Molecular biology of steroid hormone synthesis. Endocr Rev 9: 295–318.
Nakajin S, Shivley JE, Yuan P, Hall PF 1981 Microsomal cytochrome P450 from neonatal pig testis: two enzymatic activities (17 α-hydroxylase and c 17:20 lyase) associated with one protein. Biochemistry 20: 4037–4042.
Zuber MX, Simpson ER, Waterman MR 1986 Expression of bovine 17 hydroxylase cytochrome P450 cDNA in non-steroidogenic (COS 1) cells. Science 234: 1258–1260.
Fevold HR, Lorence MC, McCarthy JL, Trant JM, Kagimoto M, Waterman MR, Mason JI 1989 Rat P 450-17α from testis characterization of a full-length cDNA encoding a unique steroid hydroxylase capable of catalyzing both δ4- and δ5- steroid-17,20 lyase reactions, Mol Endocrinol 3: 968–975.
McAllister JM, Kevin JFP, Trant JM, Estabrook RW, Mason JI, Waterman MR, Simpson ER 1989 Regulation of cholesterol side-chain cleavage and 17α-hydroxylase/lyase activities in proliferating human theca interna cells in long-term monolayer culture. Endocrinology 125: 1959–1966.
Lin D, Harikrishna JA, Moore CCD, Jones KL, Miller WL 1991 Missense mutation Ser106→Pro causes 17α-hydroxylase deficiency. J Biol Chem 266: 15992–15998.
Parker LN, Odell WD 1980 Control of adrenal androgen secretion. Endocr Rev 1: 392–410.
Onoda M, Hall PF 1982 Cytochrome b5 stimulates purified testicular microsomal cytochrome P-450 (C21 side-chain cleavage). Biochem Biophys Res Commun 108: 454–460.
Shinzawa K, Kominami S, Takemori S 1985 Studies on cytochrome P450 (P45017α, lyase) from guinea pig adrenal microsomes: dual function of a single enzyme and effect of cytochrome b5. Biochim Biophys Acta 833: 151–160.
Lin D, Black SM, Nagahama Y, Miller WI 1993 Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: contributions of serine 106 and P450 reductase. Endocrinology 132: 2498–2506.
Sakai Y, Yanase T, Hara T, Takayanagi R, Haji M, Nawata H 1994 Mechanism of abnormal production of adrenal androgens in patients with adrenocortical adenomas and carcinomas. J Clin Endocrinol Metab 78: 36–40.
Voutilainen R, Tapanainen J, Chung B-Ch, Matteson KJ, Miller WL 1986 Hormonal regulation of P450scc ( 20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J Clin Endocrinol Metab 63: 202–207.
Luu The V, Lachance Y, Labrie C, Leblanc G, Thomas JL, Strickler RC, Labrie F 1989 Full length cDNA structure and deduced aminoacid sequence of human 3β-hydroxy-5-ene steroid dehydrogenase. Mol Endocrinol 3: 1310–1312.
Feinberg AP, Vogelstein B 1984 A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity (addendum). Anal Biochem 137: 266–267.
Peng C, Huang Th, Yeung E-B, Donaldson CJ, Vale WW, Leung PCK 1993 Expression of the type II activin receptor gene in the human placenta. Endocrinology 133: 3046–3049.
Sambrook J, Fritsch EF, Maniatis T 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 46–52.
Rheaume E, Lachance Y, Zhao HF, Breton N, Dumont M, Launoit Y, Trudel C, Luu-The V, Simard J, Labrie F 1991 Structure and expression of a new complementary DNA encoding the almost exclusive 3-hydroxy steroid dehydrogenase/5-4-isomerase in human adrenals and gonads. Mol Endocrinol 5: 1147–1157.
McAllister JA, Hornsby PJ 1988 Dual regulation of 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, and dehydroepiandrosterone sulfotransferase by adenosine 3′,5′-monophosphate and activators of protein kinase C in cultured human adrenocortical cells. Endocrinology 122: 2012–2018.
Cheung CY, Flasch MV, Hornsby PJ 1992 Expression of 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in fetal human adrenocortical cells transfected with SV40 T antigen. J Mol Endocrinol 9: 7–17.
Jaffe RB, Serrón-Ferré M, Crickard K, Koritink D, Mitchell BF, Huhtaniemi IT 1981 Regulation and function of the primate fetal adrenal gland and gonad. Recent Prog Horm Res 37: 41–103.
Voutilainen R, Ilvesmäki Miettinen P 1991 Low expression of 3β-hydroxy-5-ene steroid dehydrogenase gene in human fetal adrenals in vivo: adrenocorticotropin and protein kinase C-dependent regulation in adrenocortical cultures. J Clin Endocrinol Metab 72: 761–767.
Mesiano S, Coulter CL, Jaffe RB 1993 Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17,20-lyase, and 3β-hydroxysteroid dehydrogenase isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: reappraisal of functional zonation. J Clin Endocrinol Metab 77: 1184–1189.
Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ 1996 The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3 β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab 81: 3558–3565.
Gell JS, Atkins B, Margraf L, Mason JI, Sasano H, Rainey WE, Carr BR 1996 Adrenarche associated with decreased 3β-hydroxysteroid dehydrogenase expression in the adrenal reticularis. Endocr Res 22: 723–728.
Lebrethon MC, Jaillard C, Naville D, Bégeot M, Saez JM 1994 Effects of transforming growth factor-β1 on human adrenocortical fasciculata-reticularis cell differentiated functions. J Clin Endocrinol Metab 79: 1033–1039.
Miller WL, Auchus RJ, Geller DH 1997 The regulation of 17,20 lyase activity. Steroids 62: 133–142.
Morel Y, Mébarki F, Rhéaume E, Sanchez R, Forest MG, Simard J 1997 Structure-function relationships of 3 β-hydroxysteroid dehydrogenase: contribution made by the molecular genetics of 3 β-hydroxysteroid dehydrogenase deficiency. Steroids 62: 176–184.
Thomas JL, Berko EA, Faustino A, Myers RP, Strickler RC 1988 Human placental 3β-hydroxy-5-ene-steroid dehydrogenase and steroid 5→4-ene-isomerase: purification from microsomes, substrate kinetics, and inhibition by product steroids. J Steroid Biochem 31: 785–793.
Sauer LA, Chapman JC, Dauchy RT 1994 Topology of 3 β-hydroxy-5-ene-steroid dehydrogenase/δ5-δ4- isomerase in adrenal cortex mitochondria and microsomes. Endocrinology 134: 751–759.
Cherradi N, Defaye G, Chambaz EM 1994 Characterization of the 3β-hydroxysteroid dehydrogenase activity associated with bovine adrenocortical mitochondria. Endocrinology 134: 1358–1364.
Acknowledgements
The authors thank Dr. W. Miller for his methodologic suggestions. The collaboration of Sebastian Oknaian and Elina Feinstein is acknowledged.
Author information
Authors and Affiliations
Additional information
Supported by Agencia Nacional de Promocion Científica y Technologica of Argentina, Grant PID PMT-SID 0006, and Grant PMT-PICT 0028 and by the World Health Organization.
Rights and permissions
About this article
Cite this article
Dardis, A., Saraco, N., Rivarola, M. et al. Decrease in the Expression of the 3β-Hydroxysteroid Dehydrogenase Gene in Human Adrenal Tissue during Prepuberty and Early Puberty: Implications for the Mechanism of Adrenarche. Pediatr Res 45, 384–388 (1999). https://doi.org/10.1203/00006450-199903000-00016
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199903000-00016
This article is cited by
-
Adrenal changes associated with adrenarche
Reviews in Endocrine and Metabolic Disorders (2009)
-
Expression of the IGF and the aromatase/estrogen receptor systems in human adrenal tissues from early infancy to late puberty: Implications for the development of adrenarche
Reviews in Endocrine and Metabolic Disorders (2009)