Abstract
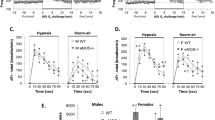
Endothelin-converting-enzyme (ECE-1) catalyzes the proteolytic activation of big endothelin-1 to mature endothelin-1. Most homozygous ECE-1−/− embryos die in utero and show severe craniofacial, enteric, and cardiac malformations precluding ventilatory function assessment. In contrast, heterozygous ECE-1+/− embryos develop normally. Their respiratory function at birth has not been studied. Taking into account previous respiratory investigations in mice with endothelin-1 gene disruption, we hypothesized that ECE-1-deficient mice may have impaired ventilatory control. We analyzed ventilatory responses to hypercapnia (8% CO2) and hypoxia (10% O2) in newborn and adult mice heterozygous for ECE-1 deficiency (ECE-1+/−) and in their wild-type littermates (ECE-1+/+). Ventilation, breath duration, and tidal volume were measured using whole-body plethysmography. Ventilatory responses to hypoxia were significantly weaker in ECE-1+/− than in ECE-1+/+ newborn mice (percentage ventilation increase: 1 ± 25%versus 33 ± 29%, p = 0.010). Baseline breathing variables and ventilatory responses to hypercapnia were normal in the ECE-1+/− newborn mice. No differences were observed between adult ECE-1+/− and ECE-1+/+ mice. We conclude that ECE-1 is required for normal ventilatory response to hypoxia at birth.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CCHS:
-
congenital central hypoventilation syndrome
- ECE-1:
-
endothelin-converting-enzyme-1
- ET-1:
-
endothelin-1
- GDNF:
-
glial-cell-line-derived neurotrophic factor
- HSCR:
-
Hirschsprung’s disease
- NTS:
-
nucleus tractus solitarius
- RET:
-
rearranged during transfection
- TTOT:
-
breath duration
- VE:
-
ventilation
- VT:
-
tidal volume
REFERENCES
Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, de Wit D, Yanagisawa M 1994 ECE-1 a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78: 473–485
Kuwaki TH, Kurihara H, Cao WH, Kurihara Y, Unekawa M, Yazaki Y, Kumada M 1997 Physiological role of brain endothelin in the central autonomic control: from neuron to knockout mouse. Prog Neurobiol 51: 545–579
Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, de Wit D, Emoto N, Hammer R 1998 Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development 125: 825–836
Kuwaki L, Ling GY, Onodera M, Ishii T, Nakamura A, Ju KH, Cao WH, Kumada M, Kurihara H, Kurihara Y, Yazaki Y, Ohuchi T, Yanagisawa M, Fukuda Y 1999 Endothelin in the central control of cardiovascular and respiratory functions. Clin Exp Pharmacol Physiol 26: 989–994
Kuwaki T, Cao WH, Kurihara Y, Kurihara H, Ling GY, Onodera M 1996 Impaired ventilatory responses to hypoxia and hypercapnia in mutant mice deficient in endothelin-1. Am J Physiol 270: R1279–R1286
Ronca AE, Abel RA, Alberts JR 1996 Perinatal stimulation and adaptation of the neonate. Acta Paediatr 416: 8–15
Hogan B, Beddington R, Constantini F, Lacy E 1994 Manipulating the Mouse Embryo: Laboratory Manual. Cold Spring Harbor Laboratory Press, Plainview, NY, pp 1–497
Drorbaugh JE, Fenn WO 1955 A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–86
Epstein MAF, Epstein RA 1978 A theoretical analysis of the barometric method for measurement of tidal volume. Respir Physiol 32: 105–120
Mortola JP, Frappell PB 1998 On the barometric method for measurements of ventilation, and its use in small animals. Can J Physiol Pharmacol 76: 937–944
Enhorning G, van Schaik S, Lundgren C, Vargas I 1998 Whole-body plethysmography, does it measure tidal volume of small animals? Can J Physiol Pharmacol 76: 945–961
Onodera M, Kuwaki T, Kumada M, Masuda Y 1997 Determination of ventilatory volume in mice by whole-body plethysmography. Jpn J Physiol 47: 317–326
Dauger S, Renolleau S, Vardon G, Népote V, Mas C, Simonneau M, Gaultier C, Gallego J 1999 Ventilatory responses to hypercapnia and hypoxia in Mash-1 heterozygous newborn and adult mice. Pediatr Res 46: 535–542
Dauger S, Nsegbe E, Vardon G, Gaultier C, Gallego J 1998 The effects of restraint on ventilatory responses to hypercapnia and hypoxia in mice. Respir Physiol 112: 215–225
Tankersley CG, Fitzgerald RS, Mitzner WA, Kleeberger SR 1993 Hypercapnic ventilatory responses in mice differentially susceptible to acute ozone exposure. J Appl Physiol 75: 2613–2619
Tankersley CG, Fitzgerald RS, Levitt RC, Mitzner WA, Ewart SL, Kleeberger SR 1997 Genetic control of differential baseline breathing pattern. J Appl Physiol 82: 874–881
Tankersley CG, Fitzgerald RS, Kleeberger SR 1994 Differential control of ventilation among inbred strains of mice. Am J Physiol 267: R1371–R1377
Tankersley CG, Kleeberger SR, Russ B, Schwartz A, Smith P 1996 Modified control of breathing in genetically obese (ob/ob) mice. J Appl Physiol 81: 716–723
Pepelko WE, Dixon GA 1975 Arterial blood gas in conscious rats exposed to hypoxia, hypercapnia, or both. J Appl Physiol 38: 581–587
Powell FL, Wilson WK, Mitchell GS 1998 Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134
Bach KB, Mitchell GS 1998 Hypercapnia-induced long-term depression of respiratory activity requires α2-adrenergic receptors. J Appl Physiol 84: 2099–2105
Bissonnette JM 2000 Mechanisms regulating hypoxic respiratory depression during fetal postnatal life. Am J Physiol 278: R1391–R1400
Eden GJ, Hanson MA 1987 Maturation of the respiratory response to acute hypoxia in the newborn rat. J Physiol 392: 1–9
Brophy S, Ford TW, Carey M, Jones JFX 1999 Activity of aortic chemoreceptors in the anaesthetized rat. J Physiol 514: 821–828
Howe A, Pack RJ, Wise JCM 1981 Arterial chemoreceptor-like activity in the abdominal vagus of the rat. J Physiol 320: 309–318
Horn EM, Waldrop TG 1999 Oxygen-sensing neurons in the caudal hypothalamus: their role in cardiorespiratory control. Respir Physiol 110: 219–228
Hilton SM 1982 The defence-arousal system its relevance for circulatory respiratory control. J Exp Biol 100: 159–174
Gulati A, Rebello S, Chari G, Bhat R 1992 Ontogeny of endothelin and its receptors in rat brain. Life Sci 51: 1715–1724
Iyer RS, Singh G, Rebello S, Roy S, Bhat R, Vidyasagar D, Gulati A 1995 Changes in the concentration of endothelin-1 during development of hypertensive rats. Pharmacology 51: 96–104
Burton MD, Kawashima A, Brayer JA, Kazemi H, Shannon DC, Schuchardt A, Costantini F, Pachnis V, Kinane TB 1997 RET proto-oncogene is important for the development of respiratory CO2 sensitivity. J Auton Nerv Syst 63: 137–143
Clouthier DE, Hosoda K, Richardson JA, Williams SC, Yanagisawa H, Kuwaki T, Kumada M, Hammer RE, Yanagisawa M 1998 Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125: 813–824
Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM 1996 Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci 16: 5361–5371
Gozal D, Simakajornboon N, Czapla MA, Xue YD, Gozal E, Vlasic V, Lasky JA, Liu JY 2000 Brainstem activation of platelet-derived growth factor-receptor modulates the late phase of the hypoxic ventilatory response. J Neurochem 74: 310–319
Grasemann H, Lu B, Jiao A, Boudreau J, Gerard NP, De Sanctis GT 1999 Targeted deletion of the neutral endopeptidase gene alters ventilatory responses to acute hypoxia in mice. J Appl Physiol 87: 1266–1271
Kline DD, Yang T, Huang PL, Prabhakar NR 1998 Altered respiratory responses to hypoxia in mutant mice deficient in neuronal nitric oxide synthase. J Physiol 511: 273–287
Gozal D 1998 Congenital central hypoventilation syndrome: an update. Pediatr Pulmonol 26: 273–282
Hofstra RM, Valdenaire O, Arch E, Osinga J, Kroes H, Loffler BM, Hamosh A, Meijers C, Buys CH 1999 A loss-of-function mutation in the endothelin-converting enzyme (ECE-1) associated with Hirschsprung disease, cardiac defects, autonomic dysfunction. Am J Hum Genet 64: 304–308
Amiel J, Salomon R, Attié T, Pelet A, Trang H, Mokhtari M, Gaultier C, Munnich A, Lyonnet S 1998 Mutations of the RET-GDNF signaling pathway in Ondine’s curse. Am J Hum Genet 62: 715–717
Sakai T, Wakizaka A, Matsuda H, Nirasawa Y, Itoh Y 1998 Point mutation in exon 12 of the receptor tyrosine kinase proto-oncogene RET in Ondine-Hirschsprung syndrome. Pediatrics 101: 924–925
Bolk S, Angrist M, Xie J, Yanagisawa M, Silvestri JM, Weese-Mayer DE, Chakravarti A 1996 Endothelin-3 frameshift mutation in congenital central hypoventilation syndrome. Nat Genet 13: 395–396
Valdenaire O, Rohrbacher E, Mattei MG 1995 Organization of the gene encoding the human-endothelin-converting enzyme (ECE-1). J Biol Chem 270: 29794–29798
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Fondation Pour La Recherche Médicale (grant awarded to S.R.) and by Université Paris VII (Legs Poix).
Rights and permissions
About this article
Cite this article
Renolleau, S., Dauger, S., Vardon, G. et al. Impaired Ventilatory Responses to Hypoxia in Mice Deficient in Endothelin-Converting-Enzyme-1. Pediatr Res 49, 705–712 (2001). https://doi.org/10.1203/00006450-200105000-00016
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-200105000-00016