Abstract
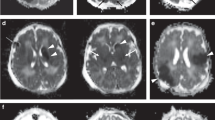
This study was undertaken to clarify whether seizures in the newborn cause damage to the healthy brain and, more specifically, to determine the extent to which seizures may contribute to the brain-damaging effects of hypoxia-ischemia (HI). Seizures were induced in 10-d-old rat pups with kainic acid (KA). Seizure duration was determined electrographically. HI was induced by common carotid artery ligation followed by exposure to 8% oxygen for either 15 or 30 min. Six groups of animals were assessed:1) controls [neither KA nor HI (group I)];2) group II, KA alone;3) group III, 15 min HI alone;4) group IV,15 min HI plus KA;5) group V, 30 min HI alone; and 6) group VI, 30 min HI plus KA. Animals were assessed neuropathologically at 3 (early) and 20 (late) d of recovery. KA injection without hypoxia resulted in continuous clinical and electrographic seizures lasting a mean of 282 min. No neuropathologic injury was seen in groups I (no HI or KA), II (KA alone), III (15 min HI alone), or IV (15 min HI and KA). Animals in group V (30 min HI alone) displayed brain damage with a mean score of 2.3 and 0.60 at 3 and 20 d of recovery, respectively. Animals in group VI (30 min HI and KA) had a mean score of 12.1 and 3.65 at 3 and 20 d of recovery, respectively. Compared with group V, the increased damage as a result of the seizure activity in group VI occurred exclusively in the hippocampus. Status epilepticus in the otherwise “healthy” neonatal brain does not cause neuropathologic injury. However, seizures superimposed on HI significantly exacerbate brain injury in a topographically specific manner.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- KA:
-
kainic acid
- HI:
-
hypoxia-ischemia
- GFAP:
-
glial fibrillary acidic protein
References
Holden KR, Mellits ED, Freeman JM 1982 Neonatal seizures I. Correlation of prenatal and perinatal events with outcomes. Pediatrics 70: 165–176
Scher MS, Kosaburo A, Beggarly ME, Hamid MY, Steppe DA, Painter MJ 1993 Electrographic seizures in preterm and fullterm neonates: clinical correlates, associated brain lesions, and risk of neurological sequelae. Pediatrics 91: 128–134
Yager JY, Vannucci RC 1997 Neonatal seizures. In: Fanaroff AA, Martin RI (eds) Neonatal-Perinatal Medicine, 6th Ed. Mosby-Year Book, Inc., Philadelphia, pp 1891–1911
Camfield PR 1997 Recurrent seizures in the developing brain are not harmful. Epilepsia 38: 735–737
Wasterlain CG 1997 Recurrent seizures in the developing brain are harmful. Epilepsia 38: 728–734
Holmes GL 1997 Epilepsy in the developing brain: lessons for the laboratory and clinic. Epilepsia 38: 12–30
Mizrahi EM, Kellaway P 1998 Diagnosis and Management of Neonatal Seizures. Lippincott-Raven, Piladelphia, pp 47–60
Sarnat HB, Sarnat MS 1976 Neonatal encephalopathy following fetal distress—A clinical and electroencephalographic study. Arch Neurol 33: 695–706
Volpe JJ 1995 Neurology of the Newborn, 3rd Ed. WB Saunders, Philadelphia, pp 172–207
Rice JE, Vannucci RC, Brierly JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Towfighi J, Yager JY, Housman C, Vannucci RC 1991 Neuropathology of remote hypoxic-ischemic damage in the immature rat. Acta Neuropathol 81: 578–587
Towfighi J, Zec N, Yager J, Housman C, Vannucci RC 1995 Temporal evolution of neuropathologic changes in an immature rat model of cerebral hypoxia: a light microscopic study. Acta Neuropathol 90: 375–386
Yager JY, Towfighi J, Vannucci RC 1993 Influence of mild hypothermia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 34: 525–529
Yager JY, Asselin J 1996 The effect of mild hypothermia on cerebral energy metabolism during evolution of hypoxic-ischemic brain damage in the immature rat. Stroke 27: 919–926
Snead OC, Stephens HI 1983 Ontogeny of cortical and subcortical electroencephalographic events in unrestrained neonatal and infant rats. Exp Neurol 82: 249–269
Mizrahi EM, Kellaway P 1987 Characterization and classification of neonatal seizures. Neurology 37: 1837–1844
Cataltepe O, Vannucci RC, Hietgan DF, Towfighi J 1995 Effect of status epilepticus on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 38: 251–257
Yager JY, Heitjan DF, Towfighi J, Vannucci RC 1992 Effect of insulin induced and fasting hypoglycemia on perinatal hypoxic-ischemic brain damage. Pediatr Res 31: 138–142
Albala BJM, Moshe SL, Okada R 1984 Kainic acid induced seizures: a development study. Dev Brain Res 13: 139–148
Nitecka L, Tremblay E, Charton G, Bouillot JP, Berger ML, Ben-Ari Y 1984 Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience 13: 1073–1094
Stafstrom CE, Thompson JL, Holmes GL 1992 Kainic acid seizures in the developing brain: status epilepticus and spontaneous recurrent seizures. Dev Brain Res 65: 227–236
Stafstrom CD, Holmes GL, Chronopoulos A, Thurber S, Thompson JL 1993 Age-dependent cognitive and behavior deficits following kainic acid-induced seizures. Epilepsia 33: 420–432
Thurber S, Chronopoulos A, Stafstrom CE, Holmes GL 1992 Behavioral effects of continuous hippocampal stimulation in the developing rat. Dev Brain Res 68: 35–40
Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L 1987 The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res 465: 43–58
Hirsch E, Baram TZ, Snead OC 1992 Ontogenic study of lithium-pilocarpine-induced status epilepticus in rats. Brain Res 583: 120–126
Liu Z, Gatt A, Werner SJ, Mikati M, Holmes GL 1995 Long-term behavioral deficits following pilocarpine-induced seizures in immature rats. Epilepsy Res 19: 191–204
Holmes GL, Gairsa J-L, Chevassus-Au-Louis N, Ben-Ari Y 1998 Consequence of neonatal seizures in the rat: morphological and behavioral effects. Ann Neurol 44: 845–857
Franck JE, Schwartzkroin PA 1984 Immature rabbit hippocampus is damaged by systemic but not intraventricular kainic acid. Dev Brain Res 13: 219–227
Dobbing J, Sands J 1979 Comparative aspects of the brain growth spurt. Early Human Dev 3: 79–83
Hagberg H, Bona E, Gilland E, Puka-Sundvall M 1997 Hypoxia-ischaemia model in the 7-day old rat: possibilities and shortcomings. Acta Paediatr Suppl 422: 85–88
Campochiaro P, Coyle JT 1978 Ontogenic development of kainate neurotoxicity: correlates with glutamatergic innervation. Proc Natl Acad Sci USA 75: 2025–2029
Rothe F, Schmidt W, Wolf G 1983 Postnatal changes in the activity of glutamate dehydrogenase and aspartate aminotransferase in the rat nervous system with special reference to the glutamate transmitter metabolism. Dev Brain Res 11: 67–74
Bickler PE, Gallego SM, Hansen BM 1993 Developmental changes in intracellular calcium regulation in rat cerebral cortex during hypoxia. J Cereb Blood Flow Metab 13: 811–819
Carmant L, Liu Z, Werner SJ, Mikati MA, Holmes GL 1995 Effect of kainic acid-induced status epilepticus on inositol-triphosphate and seizure-induced brain damage in mature and immature animals. Dev Brain Res 89: 67–72
Sperber EF, Weireter KK, Kubova H, Romero MT 1995 Age-related changes in parvalbumin immunoreactivity following kainic acid seizure. Soc Neurosci Abstr 21: 1473
Young RSK, Osbakken MD, Briggs RW, Yagel SK, Rice DW, Goldberg S 1985 31P NMR study of cerebral metabolism during prolonged seizures in the neonatal dog. Ann Neurol 18: 14–20
Young RSK, Petroff OAC, Chen B, Gore JC, Aquila WJ 1991 Brain energy state and lactate metabolism during status epilepticus in the neonatal dog. In vivo 31P and 1H nuclear magnetic resonance study. Pediatr Res 29: 191–195
Yager JY, Brucklacher RM, Vannucci RC 1992 Cerebral energy metabolism during hypoxia-ischemia and early recovery in immature rats. Am J Physiol 262: H672–H677
Vannucci RC 1990 Experimental biology of cerebral hypoxia-ischemia: relation to perinatal brain damage. Pediatr Res 27: 317–326
Yager JY, Kala G, Hertz L, Juurlink BHJ 1994 Correlation between ATP content and hypoxic-ischemic damage in immature and mature astrocytes. Dev Brain Res 82: 62–68
Jurkowitz-Alexander MS, Altschuld RA, Hohl CM, Johnson JD, McDonald JS, Simmons TD, Horrocks 1992 Cell swelling, blebbing, and death are dependent on ATP depletion and independent of calcium during chemical hypoxia in a glial cell line (ROC-1). J Neurochem 59: 344–352
Lipton P, Whittingham TS 1982 Reduced ATP concentration as a basis for synaptic transmission failure during hypoxia in the in-vitro guinea-pig hippocampus. J Physiol Lond 325: 51–65
Novelli A, Reilly JA, Lysko PG, Henneberry RC 1988 Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Res 451: 205–212
Chapman AG, Meldrum BS, Siesjo BK 1977 Cerebral metabolic changes during prolonged epileptic seizures in rats. J Neurosci 28: 1025–1035
Palmer C, Brucklacher RM, Christensen MA, Vannucci RC 1990 Carbohydrate and energy metabolism during the evolution of hypoxic-ischemic brain damage in the immature rat. J Cereb Blood Flow Metab 10: 227–235
Siesjo BK, Ingvar M, Wieloch T 1986 Cellular and molecular events underlying epileptic brain damage. Ann NY Acad Sci 462: 208–223
Jensen FE, Applegate CK, Holtzman D, Belin TR, Burchfiel JL 1991 Epileptogenic effect of hypoxia in the immature rodent brain. Ann Neurol 29: 629–637
Jensen FE, Wang C, Stafstrom CE, Liu Z, Geary C, Stevens MC 1998 Acute and chronic increases in excitability in rat hippocampal slices after perinatal hypoxia in vivo. J Neurophysiol 79: 73–81
Sato K, Morimoto K, Ujike H, Yamada T, Yamada N, Kuroda S, Hayabara T 1994 The effects of perinatal anoxia or hypoxia on hippocampal kindling development in rats. Brain Res Bull 35: 167–170
Young RSK, During MJ, Aquila WJ, Tendler D, Ley E 1992 Hypoxia increases extracellular concentrations of excitatory and inhibitory neurotransmitters in subsequently induced seizures: in vivo microdialysis study in the rabbit. Exp Neurol 117: 204–209
Busto R, Dietrich D, Globus MY-T, Ginsberg MD 1989 The importance of brain temperature in cerebral ischemic injury. Stroke 20: 1113–1114
Chen H, Chopp M, Welch KMA 1991 Effect of mild hyperthermia on the ischemic infarct volume after middle cerebral artery occlusion in the rat. Neurology 41: 1133–1135
Castillo J, Davalos A, Marrugat J, Noya M 1998 Timing for fever-related brain damage in acute ischemic stroke. Stroke 29: 2455–2460
Schwab S, Spranger M, Aschoff A, Steiner T, Hacke W 1997 Brain temperature monitoring and modulation in patients with severe MCA infarction. Neurology 48: 762–767
Jorgenson HS, Reith J, Pedersen PM, Hakayama H, Olsen TS 1996 Body temperature and outcome in stroke patients. Lancet 348: 193
Berger ML, Tremblay E, Nitecka L, Ben-Ari Y 1984 Maturation of kainic acid seizure-brain damage syndrome in the rat. III. Postnatal development of kainic acid binding sites in the limbic system. Neuroscience 13: 1095–1104
Tremblay E, Nitecka L, Berger ML, Ben-Ari Y 1984 Maturation of kainic acid seizure-brain damage syndrome in the rat. I. Clinical, electrographic and metabolic observations. Neuroscience 13: 1051–1072
Hattori H, Wasterlain CG 1990 Excitatory amino acids in the developing brain: ontogeny, plasticity, and excitotoxicity. Pediatr Neurol 6: 219–228
Tremblay E, Roisin MP, Represa A, Charriaut-Marlangue C, Ben-Ari Y 1988 Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res 461: 393–396
Moshe SL 1993 Seizures in the developing brain. Neurology 43( suppl 5): S3–S7
Owens J, Robbins CA, Wenzel HJ, Schwartzkroin PA 1997 Acute and chronic effects of hypoxia on the developing hippocampus. Ann Neurol 41: 187–199
Jensen F, Tsuji M, Offutt M, Firkusny I, Holtsman D 1993 Profound, reversible energy loss in the hypoxic immature rat brain. Dev Brain Res 73: 99–105
Toth Z, Yan X-X, Haftoglou S, Ribak C, Baram TZ 1988 Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci 18: 4285–4292
VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV 1998 Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol 43: 413–426
Harvey AS, Grattan-Smith JD, Desmond PM, Chow CW, Berkovic SF 1995 Febrile seizures and hippocampal sclerosis: frequent and related findings in intractable temporal lobe epilepsy of childhood. Pediatr Neurol 12: 201–206
Robertson C, Finer N 1985 Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol 27: 473–484
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the Health Services Utilization and Research Commission of Saskatchewan, and the Toronto Hospital for Sick Children Foundation.
Rights and permissions
About this article
Cite this article
Wirrell, E., Armstrong, E., Osman, L. et al. Prolonged Seizures Exacerbate Perinatal Hypoxic-Ischemic Brain Damage. Pediatr Res 50, 445–454 (2001). https://doi.org/10.1203/00006450-200110000-00005
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-200110000-00005
This article is cited by
-
Neuroprotective therapies in the NICU in term infants: present and future
Pediatric Research (2023)
-
Difluoroboron β-diketonate polylactic acid oxygen nanosensors for intracellular neuronal imaging
Scientific Reports (2021)
-
Akut symptomatische Anfälle bei Neonaten und Einsatz des amplitudenintegrierten EEGs (aEEG)
Zeitschrift für Epileptologie (2021)
-
Pilot study of a single-channel EEG seizure detection algorithm using machine learning
Child's Nervous System (2021)
-
Investigating and managing neonatal seizures in the UK: an explanatory sequential mixed methods approach
BMC Pediatrics (2020)