Abstract
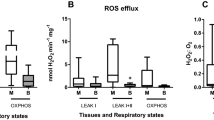
The purpose of the study was to investigate the sequence of processes occurring during and after hypoxia-induced acidemia. We used proton nuclear magnetic resonance spectroscopy, which provides an overview of metabolites in cerebrospinal fluid (CSF), reflecting neuronal metabolism and damage. The pathophysiological condition of acute fetal asphyxia was mimicked by reducing maternal uterine blood flow in 14 unanesthetized pregnant ewes. CSF metabolites were measured during hypoxia-induced acidemia, and during the following recovery period, including the periods at 24 and 48 h after the hypoxic insult. Maximum values of the following CSF metabolites were reached during severe hypoxia (pH ≤ 7.00): glucose, lactate, pyruvate, hypoxanthine, alanine, β-hydroxybutyrate, choline, creatine, myo-inositol, citrate, succinate, valine, and an unknown metabolite characterized by a resonance at 1.56 ppm in the proton nuclear magnetic resonance spectrum. Twenty-four hours after the hypoxic insult, myo-inositol was increased, and alanine was decreased 48 h after the hypoxic insult, both compared with control values. Choline levels in CSF had a linear relationship with arterial pH (r = 0.26, p < 0.005). During severe hypoxia, CSF levels of succinate and choline are increased. Increased CSF levels of succinate may indicate dysfunction of the mitochondrial respiratory chain, whereas elevated CSF choline levels may indicate disrupted cell membranes. The increase of the CSF myo-inositol level after 24 and 48 h may indicate osmolytic cell changes causing cell edema. Decreased alanine level may represent changes in the source of excitatory amino acid synthesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BEecf:
-
extracellular fluid base excess
- carotid BF:
-
carotid blood flow
- Cao2:
-
arterial oxygen content
- Cvo2:
-
venous oxygen content
- Cavo2:
-
arteriovenous difference in oxygen content
- CSF:
-
cerebrospinal fluid
- FBP:
-
fetal blood pressure
- FHR:
-
fetal heart rate
- FID:
-
free induction decay
- 1H-NMR:
-
proton nuclear magnetic resonance
- IUP:
-
intrauterine pressure
- NO:
-
nitric oxide
- Sao2:
-
arterial oxygen saturation
- Svo2:
-
venous oxygen saturation
- TCA:
-
tricarboxylic acid
- TSP:
-
trimethylsilyl-2,2,3,3-tetradeuteropropionic acid
References
van Bel F, Shadid M, Moison RM, Dorrepaal CA, Fontijn J, Monteiro L, Van de Bor M, Berger HM 1998 Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, electrical brain activity. Pediatrics 101: 185–189
Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD 1995 Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res 29: 1–14
Harkness RA 1988 Hypoxanthine, xanthine uridine in body fluids, indicators of ATP depletion. J Chromatogr 429: 255–278
Mathew OP, Bland H, Boxerman SB, James E 1980 CSF lactate levels in high risk neonates with without asphyxia. Pediatrics 66: 224–227
Hagberg H, Thornberg E, Blennow M, Kjellmer I, Lagercrantz H, Thiringer K, Hamberger A, Sandberg M 1993 Excitatory amino acids in the cerebrospinal fluid of asphyxiated infants: relationship to hypoxic-ischemic encephalopathy. Acta Paediatr 82: 925–929
Harkness RA, Lund RJ 1983 Cerebrospinal fluid concentrations of hypoxanthine, xanthine, uridine adenosine: high concentrations of the ATP metabolite, hypoxanthine, after hypoxia. J Clin Pathol 36: 1–8
Wevers RA, Engelke U, Wendel U, de Jong JG, Gabreëls FJ, Heerschap A 1995 Standardized method for high-resolution 1H-NMR of cerebrospinal fluid. Clin Chem 41: 744–751
Richardson BS, Carmichael L, Homan J, Patrick JE 1993 Cerebral oxidative metabolism in fetal sheep with prolonged graded hypoxemia. J Dev Physiol 19: 77–83
Thiringer K, Karlsson K, Rosen KG, Kjellmer I 1984 Contribution of heart muscle, liver, skeletal muscle placenta to the asphyxial hypoxanthine elevation in the acutely exteriorised fetal lamb. Biol Neonate 45: 169–182
Fog R 1992 Neuronal uridine metabolism. Acta Neurol Scand Suppl 137: 45–47
Low JA 1993 The relationship of asphyxia in the mature fetus to long-term neurologic function. Clin Obstet Gynecol 36: 82–90
De Haan HH, van Reempts JL, Vles JS, de Haan J, Hasaart TH 1993 Effects of asphyxia on the fetal lamb brain. Am J Obstet Gynecol 169: 1493–1501
Tan WK, Williams CE, During MJ, Mallard CE, Gunning MI, Gunn AJ, Gluckmann PD 1996 Accumulation of cytotoxins during the development of seizures edema after hypoxic-ischemic injury in late gestation fetal sheep. Pediatr Res 39: 791–797
Van Cappellen van Walsum AM, Jongsma HW, Wevers RA, Nijhuis JG, Crevels J, Engelke UFH, Moolenaar SH, Oeseburg B, Nijland R 2001 Hypoxia in fetal sheep: a study with1H-NMR spectroscopy of cerebrospinal fluid. Pediatr Res 49: 698–704
Ben-Yoseph O, Badar Goffer RS, Morris PG, Bachelard HS 1993 Glycerol 3-phosphate lactate as indicators of the cerebral cytoplasmic redox state in severe mild hypoxia respectively: a 13C- 31P-n.m.r. study. Biochem J 291: 915–919
Kristian T, Siesjo BK 1996 Calcium-related damage in ischemia. Life Sci 59: 357–367
Yamashita T, Kohmura E, Yamauchi A, Shimada S, Yuguchi T, Sakaki T, Miyai A, Tohyama M, Hayakawa T 1996 Induction of Na+/myo-inositol cotransporter mRNA after focal cerebral ischemia: evidence for extensive osmotic stress in remote areas. J Cereb Blood Flow Metab 16: 1203–1210
Scremin OU, Jenden DJ 1989 Focal ischemia enhances choline output decreases acetylcholine output from rat cerebral cortex. Stroke 20: 92–95
Thiringer K, Blomstrand S, Hrbek A, Karlsson K, Kjellmer I 1982 Cerebral arterio-venous difference for hypoxanthine lactate during graded asphyxia in the fetal lamb. Brain Res 239: 107–117
De Haan HH, Yzermans AC, de Haan J, Van Belle H, Hasaart TH 1994 Effects of surgery asphyxia on levels of nucleosides, purine bases, lactate in cerebrospinal fluid of fetal lambs. Pediatr Res 36: 595–600
Hope PL, Costello AM, Cady EB, Delpy DT, Tofts PS, Chu A, Reynolds EOR 1984 Cerebral energy metabolism studied with phosphorus NMR spectroscopy in normal birth-asphyxiated infants. Lancet 2: 366–370
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen-Reece H, Kirkbride V, Cooper DE, Aldridge RF, Roth SC, Brown G, Delpy DT, Reynolds EOR 1994 Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36: 699–706
Roth SC, Baudin J, Cady E, Johal K, Townsend JP, Wyatt JS, Reynolds EO, Stewart AL 1997 Relation of deranged neonatal cerebral oxidative metabolism with neurodevelopmental outcome head circumference at 4 years. Dev Med Child Neurol 39: 718–725
Thordstein M, Bågenholm R, Thiringer K, Kjellmer I 1993 Scavengers of free oxygen radicals in combination with magnesium ameliorate perinatal hypoxic-ischemic brain damage in the rat. Pediatr Res 34: 23–26
Williams CE, Gunn A, Gluckman PD 1991 Time course of intracellular edema epileptiform activity following prenatal cerebral ischemia in sheep. Stroke 22: 516–521
Erecineska M, Nelson D, Nissim I, Daikhin Y, Yudkoff M 1994 Cerebral alanine transport alanine aminotransferase reaction: alanine as a source of neuronal glutamate. J Neurochem 62: 1953–1964
Griffin JL, Rae C, Dixon RM, Radda GK, Matthews PM 1998 Excitatory amino acid synthesis in hypoxic brain slices: does alanine act as a substrate for glutamate production in hypoxia?. J Neurochem 71: 2477–2486
Acknowledgements
The authors thank Theo Arts and Alex Hanssen of the Central Animal Laboratory Nijmegen, and Sjaak van Asten for their assistance. 1H-NMR spectra were recorded at the Dutch hf-NMR facility at the Department of Biophysical Chemistry, University of Nijmegen, The Netherlands (department head, Prof. C.W. Hilbers). We also thank J. Joordens for his invaluable help and assistance. Furthermore, we gratefully acknowledge the help of K. Wethly and A. Stegeman of the Laboratory for Pediatrics and Neurology for the measurement of the blood samples for purines.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Nellcor Puritan Bennett Inc., Pleasanton, CA, U.S.A.
Rights and permissions
About this article
Cite this article
Van Cappellen Van Walsum, AM., Jongsma, H., Wevers, R. et al. 1H-NMR Spectroscopy of Cerebrospinal Fluid of Fetal Sheep during Hypoxia-Induced Acidemia and Recovery. Pediatr Res 52, 56–63 (2002). https://doi.org/10.1203/00006450-200207000-00012
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-200207000-00012
This article is cited by
-
Simultaneous determination of lactic acid and pyruvic acid in tissue and cell culture media by gas chromatography after in situ derivatization-ultrasound-assisted emulsification microextraction
Analytical and Bioanalytical Chemistry (2019)
-
Urine metabolomic profiling of children with respiratory tract infections in the emergency department: a pilot study
BMC Infectious Diseases (2016)
-
Comprehensive 1H NMR metabolic profiling of body fluids for differentiation of meningitis in adults
Metabolomics (2016)
-
Propofol Compared with Isoflurane Inhibits Mitochondrial Metabolism in Immature Swine Cerebral Cortex
Journal of Cerebral Blood Flow & Metabolism (2014)
-
Dicholine salt of succinic acid, a neuronal insulin sensitizer, ameliorates cognitive deficits in rodent models of normal aging, chronic cerebral hypoperfusion, and beta-amyloid peptide-(25–35)-induced amnesia
BMC Pharmacology (2008)