Abstract
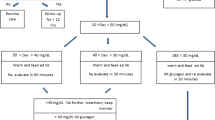
In preterm infants low plasma glucose concentrations are frequently observed. We hypothesized that the infants' ability to adapt endogenous glucose production to diminishing exogenous supply is disturbed, but will improve with increasing gestational age. Glucose production rate and gluconeogenesis were measured using stable isotope techniques with [6,6-2H2]glucose and [2-13C]glycerol in 19 preterm infants (10 ≤ 30 wk and nine >30 wk gestational age) on d 5.0 ± 1.4 of life. Exogenous glucose was administered at a rate of 33 μmol·kg−1·min−1 followed by 22 μmol·kg−1·min−1. In the first 2 h after the decrease in exogenous supply, plasma glucose concentration declined comparably in both groups: ≤30 wk, from 4.3 ± 1.2 to 3.2 ± 0.9 mM; >30 wk, from 3.7 ± 0.7 to 3.0 ± 0.6 mM. Thereafter, only in infants >30 wk an increase was observed, to 3.4 ± 0.8 mM. Glucose production rate increased comparably in both groups: ≤30 wk, from 6.0 ± 4.1 to 8.8 ± 3.4 μmol·kg−1·min−1; >30 wk, from 7.8 ± 4.6 to 11.6 ± 5.2 μmol·kg−1·min−1. This increase was equivalent to approximately 30% of the decline in exogenous glucose. Gluconeogenesis increased comparably in both groups: <30 wk, from 3.2 ± 1.2 to 4.5 ± 1.3 μmol·kg−1·min−1; >30 wk, from 4.3 ± 1.9 to 6.8 ± 2.9 μmol·kg−1·min−1. We conclude that preterm infants can only partly compensate a decline in exogenous glucose supply by increasing endogenous glucose production rate, probably because of limitations in the final common pathway of intracellular glucose metabolism (i.e. glucose-6-phosphatase). The ability to maintain the plasma glucose concentration after a decrease in exogenous supply is better preserved in infants >30 wk owing to more efficient adaptation of peripheral glucose utilization.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- AGA:
-
appropriate for gestational age
- CI:
-
confidence interval
- GPR:
-
(endogenous) glucose production rate
- MIDA:
-
mass isotopomer distribution analysis
- Ra:
-
rate of appearance
- SGA:
-
small for gestational age
References
Lubchenco LO, Bard H 1971 Incidence of hypoglycemia in newborn infants classified by birth weight and gestational age. Pediatrics 47: 831–838
De Leeuw R, de Vries IJ 1976 Hypoglycemia in small-for-dates newborn infants. Pediatrics 58: 18–22
Cornblath M, Schwartz R 1991 Disorders of carbohydrate metabolism in infancy. Blackwell Scientific Publications, Boston, pp 55–123.
Shelley HJ 1961 Glycogen reserves and their changes at birth and in anoxia. Br Med Bull 17: 137–143
Cowett RM 1998 Principles of Perinatal-Neonatal Metabolism. Springer Verlag, New York, pp 701–706, 1154–1155.
Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, Kipnis DM 1977 Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 26: 1016–1023
Cowett RM, Oh W, Schwartz R 1983 Persistent glucose production during glucose infusion in the neonate. J Clin Invest 71: 467–475
Cowett RM, Susa JB, Oh W, Schwartz R 1984 Glucose kinetics in glucose-infused small for gestational age infants. Pediatr Res 18: 74–79
Cowett RM, Wolfe RR 1991 Glucose and lactate kinetics in the neonate. J Dev Physiol 16: 341–347
Kalhan SC, Oliven A, King KC, Lucero C 1986 Role of glucose in the regulation of endogenous glucose production in the human newborn. Pediatr Res 20: 49–52
Baarsma R, Chapman TE, van Asselt WA, Berger R, Okken A 1990 Glucose kinetics in preterm and near term small-for-gestational age newborn infants. In: Chapman TE, Berger R, Reijngoud D-J, Okken A (eds) Stable Isotopes in Pediatric Nutritional and Metabolic Research. Intercept Ltd, Andover, U.K., pp 123–132.
Baarsma R, Reijngoud D-J, Berger R, Okken A 1991 Response of endogenous glucose production (EGPR) to a change in blood glucose (BG) level in low birth weight (LBW) infants on the first day of life. Pediatr Res 30: 641[ abstr]
Lafeber HN, Sulkers EJ, Chapman TE, Sauer PJJ 1990 Glucose production and oxidation in preterm infants during total parenteral nutrition. Pediatr Res 28: 153–157
Hertz DE, Karn CA, Liu YM, Liechty EA, Denne SC 1993 Intravenous glucose suppresses glucose production but not proteolysis in extremely premature newborns. J Clin Invest 92: 1752–1758
Van Goudoever JB, Sulkers EJ, Chapman TE, Carnielli VP, Efstatopoulos T, Degenhart HJ, Sauer PJ 1993 Glucose kinetics and glucoregulatory hormone levels in ventilated preterm infants on the first day of life. Pediatr Res 33: 583–589
Tyrala EE, Chen X, Boden G 1994 Glucose metabolism in the infant weighing less than 1100 grams. J Pediatr 125: 283–287
Sunehag A, Gustafsson J, Ewald U 1994 Very immature infants (≤ 30 wk) respond to glucose infusion with incomplete suppression of glucose production. Pediatr Res 36: 550–555
Sunehag A, Ewald U, Larsson A, Gustafsson J 1993 Glucose production rate in extremely immature neonates (<28 weeks) studied by use of deuterated glucose. Pediatr Res 33: 97–100
Kalhan SC, Savin SM, Adam PAJ 1976 Measurement of glucose turnover in the human newborn with glucose-1-13C. J Clin Endocrinol Metab 43: 704–707
Kalhan SC, Bier DM, Savin SM, Adam PAJ 1980 Estimation of glucose turnover and13C recycling in the human newborn by simultaneous [1-13C]glucose and [6,6-2H2]glucose tracers. J Clin Endocrinol Metab 50: 456–460
Denne SC, Kalhan SC 1986 Glucose carbon recycling and oxidation in human newborns. Am J Physiol 251: E71–E77
Sunehag A, Haymond MW, Schanler RJ, Reeds PJ, Bier DM 1999 Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes 48: 791–800
Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC 1995 Use of 2H2O for estimating rates of gluconeogenesis. J Clin Invest 95: 172–178
Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC 1996 Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 98: 378–385
Ackermans MT, Pereira Arias AM, Bisschop PH, Endert E, Sauerwein HP, Romijn JA 2001 Rates of gluconeogenesis in healthy men measured with 2H2O are higher than measured with [2-13C]glycerol. J Clin Endocrinol Metab 86: 2220–2226
Wolfe RR, Allsop JR, Burke JF 1979 Glucose metabolism in man: responses to intravenous glucose infusion. Metabolism 28: 210–220
Kloosterman GJ 1970 On intrauterine growth. Int J Gynaecol Obstet 8: 895–912
Dekker E, Hellerstein MK, Romijn JA, Neese RA, Peshu N, Endert E, Marsh K, Sauerwein HP 1997 Glucose homeostasis in children with falciparum malaria: precursor supply limits gluconeogenesis and glucose production. J Clin Endocrinol Metab 82: 2514–2521
Schutgens RBH, Tjoe Awie C, Beemer FA, Berntssen WJM 1977 Rapid enzymic micromethod for the quantitative determination ofl(+)alanine in blood and urine. Clin Chim Acta 80: 1–5
Wolfe RR 1992 Calculations of substrate kinetics: single pool model. In: Wolfe RR (ed) Radioactive and Stable Isotope Tracers in Biomedicine. Principles and Practice of Kinetic Analysis. Wiley-Liss, New York, pp 119–144.
Hellerstein MK, Neese RA 1992 Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. Am J Physiol 263: E988–E1001
Neese RA, Schwartz J-M, Faix D, Turner S, Letscher A, Vu D, Hellerstein MK 1995 Gluconeogenesis and intrahepatic triose phosphate flux in response to fasting or substrate loads. J Biol Chem 270: 14452–14466
Mestyán J, Soltész G, Schultz K, Horváth M 1975 Hyperaminoacidemia due to the accumulation of gluconeogenic amino acid precursors in hypoglycemic small-for-gestational age infants. J Pediatr 87: 409–414
Haymond MW, Karl IE, Pagliara AS 1974 Increased gluconeogenic substrates in the small-for-gestational-age infant. N Engl J Med 291: 322–328
Stanley CA, Anday EK, Baker L, Delivoria-Papadopolous M 1979 Metabolic fuel and hormone responses to fasting in newborn infants. Pediatrics 64: 613–619
Williams PR, Fiser RH, Sperling MA, Oh W 1975 Effects of oral alanine feeding on blood glucose, plasma glucagon and insulin concentrations in small-for-gestational-age infants. N Engl J Med 292: 612–614
Marsac C, Saudubray JM, Moncion A, Leroux JP 1976 Development of gluconeogenic enzymes in the liver of human newborns. Biol Neonate 28: 317–325
Shelley HJ, Neligan GA 1966 Neonatal hypoglycaemia. Br Med Bull 22: 34–39
Girard J, Ferré P, Pégorier JP, Duée PH 1992 Adaptations of glucose and fatty acid metabolism during perinatal period and suckling-weaning transition. Physiol Rev 72: 507–562
Devi BG, Habeebullah CM, Gupta PD 1992 Glycogen metabolism during human liver development. Biochem Int 28: 229–237
Corssmit EPM, Stouthart JML, Romijn JA, Endert E, Sauerwein HP 1994 Sex differences in the adaptation of glucose metabolism to short term fasting: effects of oral contraceptives. Metabolism 43: 1503–1508
Hume R, Burchell A 1993 Abnormal expression of glucose-6-phophatase in preterm infants. Arch Dis Child 68: 202–204
Marangou AG, Alford FP, Ward G, Liskaser F, Aitken PM, Weber KM, Boston RC, Best JD 1988 Hormonal effects of norepinephrine on acute glucose disposal in humans: a minimal model analysis. Metabolism 37: 885–891
Boden G 1997 Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes 46: 3–10
Acknowledgements
The authors thank the participating children, their parents, and the medical and nursing staff of both neonatology departments for their cooperation. We thank G.J. Weverling, Ph.D., Department of Clinical Epidemiology and Biostatistics, Academic Medical Center, Amsterdam, for his help with the statistical analysis.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Van Kempen, A., Romijn, J., Ruiter, A. et al. Adaptation of Glucose Production and Gluconeogenesis to Diminishing Glucose Infusion in Preterm Infants at Varying Gestational Ages. Pediatr Res 53, 628–634 (2003). https://doi.org/10.1203/01.PDR.0000054733.13366.AF
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000054733.13366.AF
This article is cited by
-
Hyperglycemia and prematurity: a narrative review
Pediatric Research (2023)
-
Does a reduced glucose intake prevent hyperglycemia in children early after cardiac surgery? a randomized controlled crossover study
Critical Care (2012)