Abstract
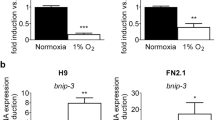
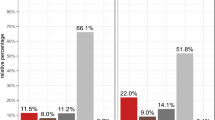
After birth, constriction of the full-term ductus arteriosus produces ischemic hypoxia, caspase activation, DNA fragmentation (>70% of cell nuclei are positive by the terminal deoxynucleotidyl transferase nick-end labeling [TUNEL] technique), and permanent ductus closure. In contrast, the preterm ductus frequently fails to develop these changes. We used the TUNEL technique to examine rings of fetal ductus arteriosus (incubated for 24 h at different oxygen and glucose concentrations) to determine the roles of 1) constriction and shortening, 2) hypoxia, and 3) hypoglycemia in producing cell death. Under controlled conditions, late-gestation ductus rings had a low rate of TUNEL-positive staining (0.6 ± 0.9%) that did not change during muscle shortening. Although hypoxia (6.9 ± 3.5%) and hypoglycemia (2.4 ± 1.9%) increased the incidence of TUNEL-positive staining, only the combination of hypoxia-plus-hypoglycemia increased the incidence to the range found in vivo (83 ± 9.5%). The combination of hypoxia-plus-hypoglycemia was associated with an oligonucleosomal pattern of DNA fragmentation. Under the same experimental conditions, the preterm ductus was capable of developing a similar degree of TUNEL-positive staining as found at term. Although caspase-3 and caspase-7 were activated in rings exposed to hypoxia-plus-hypoglycemia, a nonselective caspase inhibitor, Z-VAD.FMK (which inhibited caspase-3 and caspase-7 cleavage in the rings), did not diminish the degree of TUNEL-positive staining. We hypothesize that the preterm ductus is capable of developing an extensive degree of cell death, if it can develop the same degree of hypoxia and hypoglycemia found in the full-term newborn ductus. We also hypothesize that cell death in the ductus wall may involve pathways that are not dependent on caspase-3 or -7 activation.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- TUNEL:
-
terminal deoxynucleotidyl transferase nick-end labeling
- PARP:
-
poly (ADP-ribose) polymerase
References
Clyman RI, Mauray F, Roman C, Heymann MA, Payne B 1983 Factors determining the loss of ductus arteriosus responsiveness to prostaglandin E2 . Circulation 68: 433–436
Clyman RI, Chan CY, Mauray F, Chen YQ, Cox W, Seidner SR, Lord EM, Weiss H, Wale N, Evan SM, Koch CJ 1999 Permanent anatomic closure of the ductus arteriosus in newborn baboons: the roles of postnatal constriction, hypoxia, and gestation. Pediatr Res 45: 19–29
Slomp J, Gittenberger-de Groot AC, Glukhova MA, Conny van Munsteren J, Kockx MM, Schwartz SM, Koteliansky VE 1997 Differentiation, dedifferentiation, and apoptosis of smooth muscle cells during the development of the human ductus arteriosus. Arterioscler Thromb Vasc Biol 17: 1003–1009
Kajino H, Goldbarg S, Roman C, Liu BM, Mauray F, Chen YQ, Takahashi Y, Koch CJ, Clyman RI 2002 Vasa vasorum hypoperfusion is responsible for medial hypoxia and anatomic remodeling in the newborn lamb ductus arteriosus. Pediatr Res 51: 228–235
Kajino H, Chen YQ, Seidner SR, Waleh N, Mauray F, Roman C, Chemtob S, Koch CJ, Clyman RI 2001 Factors that increase the contractile tone of the ductus arteriosus also regulate its anatomic remodeling. Am J Physiol 281: R291–R301
Goldbarg SH, Takahashi Y, Cruz C, Kajino H, Roman C, Liu BM, Chen YQ, Mauray F, Clyman RI 2002 In utero indomethacin alters O2 delivery to the fetal ductus arteriosus: implications for postnatal patency. Am J Physiol Regul Integr Comp Physiol 282: R184–R190
Seidner SR, Chen Y-Q, Oprysko PR, Mauray F, Tse MM, Lin E, Koch C, Clyman RI 2001 Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res 50: 365–373
Majno G, Joris I 1995 Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol 146: 3–15
Gavrieli Y, Sherman Y, Ben-Sasson SA 1992 Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 119: 493–501
Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR, Sikorska M 1993 Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J 12: 3679–3684
Cohen GM 1997 Caspases: the executioners of apoptosis. Biochem J 326: 1–16
Nunez G, Benedict MA, Hu Y, Inohara N 1998 Caspases: the proteases of the apoptotic pathway. Oncogene 17: 3237–3245
Salvesen GS, Dixit VM 1997 Caspases: intracellular signaling by proteolysis. Cell 91: 443–446
Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC 1994 Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 371: 346–347
Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin T-T, Yu VL, Miller DK 1995 Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 376: 37–43
Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, Mann M, Krammer PH, Peter ME, Dixit VM 1996 FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death–inducing signaling complex. Cell 85: 817–827
MacFarlane M, Cain K, Sun XM, Alnemri ES, Cohen GM 1997 Processing/activation of at least four interleukin-1beta converting enzyme-like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells. J Cell Biol 137: 469–479
Gastman BR, Johnson DE, Whiteside TL, Rabinowich H 1999 Caspase-mediated degradation of T-cell receptor zeta-chain. Cancer Res 59: 1422–1427
Zaks TZ, Chappell DB, Rosenberg SA, Restifo NP 1999 Fas-mediated suicide of tumor-reactive T cells following activation by specific tumor: selective rescue by caspase inhibition. J Immunol 162: 3273–3279
Kitanaka C, Kuchino Y 1999 Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ 6: 508–515
Clyman RI, Campbell D, Heymann MA, Mauray F 1985 Persistent responsiveness of the neonatal ductus arteriosus in immature lambs: a possible cause for reopening of patent ductus arteriosus after indomethacin induced closure. Circulation 71: 141–145
Narayanan M, Cooper B, Weiss H, Clyman RI 2000 Prophylactic indomethacin factors determining permanent ductus arteriosus closure. J Pediatr 136: 330–337
Grinnell F, Zhu M, Carlson MA, Abrams JM 1999 Release of mechanical tension triggers apoptosis of human fibroblasts in a model of regressing granulation tissue. Exp Cell Res 248: 608–619
Bhattacharjee R, Cowan KN, Leung WCY, Rabinovitch M 2001 Stress unloading induction of apoptosis and apoptosis mediated matrix degradation contributes to regression of pulmonary artery hypertrophy. Pediatr Res 49: 305A( abstr)
Clyman RI, Mauray F, Wong L, Heymann MA, Rudolph AM 1978 The developmental response of the ductus arteriosus to oxygen. Biol Neonate 34: 177–181
Kajino H, Chen YQ, Chemtob S, Waleh N, Koch CJ, Clyman RI 2000 Tissue hypoxia inhibits prostaglandin and nitric oxide production and prevents ductus arteriosus reopening. Am J Physiol 279: R278–R286
Guo Y, Kyprianou N 1999 Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res 59: 1366–1371
Sarin A, Wu ML, Henkart PA 1996 Different interleukin-1 beta converting enzyme (ICE) family protease requirements for the apoptotic death of T lymphocytes triggered by diverse stimuli. J Exp Med 184: 2445–2450
Xiang J, Chao DT, Korsmeyer SJ 1996 BAX-induced cell death may not require interleukin 1 beta-converting enzyme-like proteases. Proc Natl Acad Sci U S A 93: 14559–14563
Sperandio S, de Belle I, Bredesen DE 2000 An alternative, nonapoptotic form of programmed cell death. Proc Natl Acad Sci U S A 97: 14376–14381
Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM 2001 Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410: 549–554
Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL 2000 Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102: 43–53
Silke J, Hawkins CJ, Ekert PG, Chew J, Day CL, Pakusch M, Verhagen AM, Vaux DL 2002 The anti-apoptotic activity of XIAP is retained upon mutation of both the caspase 3- and caspase 9-interacting sites. J Cell Biol 157: 115–124
Wang KK 2000 Calpain and caspase: can you tell the difference?. Trends Neurosci 23: 20–26
Johnson DE 2000 Noncaspase proteases in apoptosis. Leukemia 14: 1695–1703
Kitanaka C, Kato K, Ijiri R, Sakurada K, Tomiyama A, Noguchi K, Nagashima Y, Nakagawara A, Momoi T, Toyoda Y, Kigasawa H, Nishi T, Shirouzu M, Yokoyama S, Tanaka Y, Kuchino Y 2002 Increased Ras expression and caspase-independent neuroblastoma cell death: possible mechanism of spontaneous neuroblastoma regression. J Natl Cancer Inst 94: 358–368
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported, in part, by U.S. Public Health Service National Heart, Lung, Blood Institute grants HL 46691 and HL 56061, and a gift from the Perinatal Associates Research Foundation. S.G. is a research fellow with the Stanley J. Sarnoff Endowment for Cardiovascular Research.
Rights and permissions
About this article
Cite this article
Goldbarg, S., Quinn, T., Waleh, N. et al. Effects of Hypoxia, Hypoglycemia, and Muscle Shortening on Cell Death in the Sheep Ductus Arteriosus. Pediatr Res 54, 204–211 (2003). https://doi.org/10.1203/01.PDR.0000072519.61060.E5
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000072519.61060.E5
This article is cited by
-
Pathology and molecular mechanisms of coarctation of the aorta and its association with the ductus arteriosus
The Journal of Physiological Sciences (2017)