Abstract
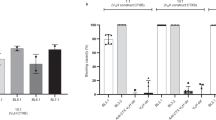
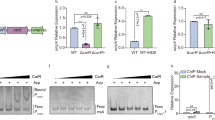
Diarrhea in infants and children is a major health hazard worldwide. Certain toxigenic diarrheas occur more commonly and are manifested more severely during the neonatal period. We have previously studied the regulation of cholera toxin–induced secretion in animal models during development. In those studies we have shown that cholera toxin stimulates a much greater secretion by immature compared with mature small intestine, and the mechanism appears to be an up-regulation of postreceptor signal transduction molecules (adenyl cyclase and Gsα) leading to an elevated cAMP level. In this study, using experimental models of human intestinal development (fetal cell lines, a micro-Ussing chamber, organ cultures, and fetal intestinal xenograft transplants), we provide preliminary evidence that cholera toxin induces an enhanced secretion mediated in part by a developmental up-regulation of the cAMP response in immature versus mature human small intestine. Additional studies are needed, however, to further define whether other developmental events (e.g. receptor expression) also regulate cholera toxin–enterocyte-enhanced interaction. Nonetheless, this approach to determining the role of development in the pathophysiology of cholera in infants may help in strategies to prevent and treat this condition and other age-related intestinal infectious diseases.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CT:
-
cholera toxin
- I sc :
-
short circuit current
- T84:
-
a human colonic cancer cell line
- H4:
-
a primary human fetal crypt small intestinal cell line
- tsHFIE:
-
a temperature-sensitive, conditionally immortalized human fetal intestinal epithelial villous cell line
- PGE2:
-
prostaglandin E2
- SCID:
-
severe combined immunodeficiency
- CFTR:
-
cystic fibrosis transmembrane conductance regulator
References
Snyder JD, Merson MH 1982 The magnitude of the global problem of acute diarrheal disease: a review of active surveillance data. Bull World Health Organ 60: 605–613
Chu SW, Walker WA 1993 Bacterial toxin interaction with the developing intestine: a possible explanation for toxogenic diarrhea of infancy. Gastroenterology 104: 916–925
Field M, Rao MC, Chang EB 1989 Intestinal electrolyte transport and diarrheal disease. N Engl J Med 321: 879–883
Chu SW, Ely IG, Walker WA 1989 Age and cortisone alter host responsiveness to cholera toxin in the developing gut. Am J Physiol 256: G220–G226
Cohen MB, Moyer MS, Luttrell M, Giannella RA 1986 The immature rat small intestine exhibits an increased sensitivity and response toEscherichia coliheat-stable enterotoxin. Pediatr Res 20: 555–560
Lencer WI, Chu SW, Walker WA 1987 Differential binding kinetics of cholera toxin to intestinal microvillus membrane during development. Infect Immun 55: 3126–3130
Seo JK, Chu SW, Walker WA 1989 Development of intestinal host defense: an increased sensitivity in the adenylate cyclase response to cholera toxin in suckling rats. Pediatr Res 25: 225–227
Raufman J-P 1997 Cholera. Am J Med 104: 386–394
Pratha VS, Thompson SM, Hogan DL, Paulis P, Dreilinger AD, Barrett KE, Isenberg JI 1998 The utility of endoscopic biopsies to quantitate the human duodenal ion transport. J Lab Clin Med 132: 512–518
Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu Z, Pringault E, Leon-Robine S, Louvard D, Walker WA 1996 Human fetal enterocytesin vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA 93: 7717–7722
Quaroni A, Beaulieu JF 1997 Cell dynamics and differentiation of conditionally immortalized human intestinal epithelial cells. Gastroenterology 113: 1198–1213
Autrup H 1980 Explant culture of human colon. Methods Cell Biol 21B: 385–401
Savidge TC, Morey AL, Ferguson DJP, Leming KA, Shmakov AN, Phillips AD 1995 Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation 56: 361–371
Ussing HH, Zerahn K 1999 Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. [Reprinted from Acta. Physiol Scand 1951;123:110–127]. J Am Soc Nephrol 10: 2056–2065
Quaroni A, Wands J, Trelstad RL, Isselbacher KJ 1979 Epithelial cell cultures from rat small intestine. J Cell Biol 80: 245–265
Buisane MP, Aubert JP, Walker WA, Savidge TC 2003 Developmental patterns of mucin gene expression in human fetal small intestinal xenografts maintained in severe-combined immunodeficient mice. Pediatr Res, in press
Nanthakumar NN, Klopcic Walker WA 2003 Normal glucocorticoid induced development of the human small intestinal xenograft. Am J Physiol ( in press)
Sanderson IR, Xu Z, Chu SW, Xie QY, Levine L, Walker WA 1996 Developmental differences in the stimulatory GTP binding protein subunit (Gsα) for adenylate cyclase in the rat small intestine. Gut 38: 853–858
Claud EC, Savidge T, Walker WA 2003 Modulation of human intestinal epithelial cell interleukin-8 secretion by human milk factors. Pediatr Res ( in press)
Nanthakumar N, Fusunyan RD, Sanderson IR, Walker WA 2000 Inflammation in the developing human intestine: a possible pathophysiologic basis for necrotizing enterocolitis. Proc Natl Acad Sci USA 97: 6043–6048
Jodal M, Lundgren O 1995 Nerves and cholera secretion. Gastroenterology 108: 287–288
Jodal M, Holmgren S, Lundgren O, Sjöqvist 1993 Involvement of the myenteric plexus in cholera toxin-induced net fluid secretion in the rat small intestine. Gastroenterology 105: 1286–1293
Mourad FH, Nassar CF 2000 Effect of vasoactive intestinal polypeptide (VIP) antagonism on rat jejunal fluid and electrolyte secretion induced by cholera andEscherichia colienterotoxins. Gut 47: 382–386
Beubler E, Kollar G, Saria A, Bukhave K, Rask-Madsen J 1989 Involvement of 5-hydroxytryptamine, prostagl and in E2 and cyclic adenosine monophosphate in cholera toxin-induced fluid secretion in the small intestine of the rat in vivo. Gastroenterology 96: 368–376
Burleigh DE, Borman RA 1997 Evidence for a nonneural electrogenic effect of cholera toxin on human isolated ileal mucosa. Dig Dis Sci 42: 1964–1968
Dominguez P, Barros F, Lazo PS 1985 The activation of adenylate cyclase from small intestinal epithelium by cholera toxin. Eur J Biochem 146: 533–538
Taylor SS 1989 cAMP-dependent protein kinase. Model for an enzyme family. J Biol Chem 264: 8443–8446
Hansson GC 1988 Cystic fibrosis and chloride-secreting diarrhoea. Nature 333: 711
Quinton PM 1999 Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev 79( suppl) S3–S22
Nath SK, Huang X, L'helgoualc'h A, Rautureau M, Bisalli A, Heyman M, Desjeux JF 1994 Relation between chloride secretion and intracellular cyclic adenosine monophosphate in a cloned human intestinal cell line HT-29 cl 19A. Gut 35: 631–636
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the National Institutes of Health T32-DK07477 (L.L.); R37-HD12437; R01-HD31852; P01-DK33506; P30-DK40561 (W.A.W.)].
Rights and permissions
About this article
Cite this article
Lu, L., Baldeon, M., Savidge, T. et al. Development of Microbial-Human Enterocyte Interaction: Cholera Toxin. Pediatr Res 54, 212–218 (2003). https://doi.org/10.1203/01.PDR.0000074974.21797.83
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000074974.21797.83