Abstract
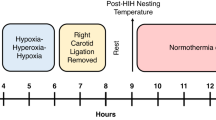
Distinctive cerebral lesions with disruptions to the developing white matter are found in very low birth weight (VLBW) infants. Although hypoxia-ischemia (HI) is a causal pathway, the pathogenesis of cerebral white matter injury in the VLBW infant is not fully understood. Pertinent murine models would facilitate the investigation of the processes leading to these cerebral lesions and enable the evaluation of therapeutic strategies. Postnatal d 3 (P3) rats are at a stage of cortical oligodendroglial maturation and axonal outgrowth similar to very preterm infants. Our aim was to characterize the effects of a focal hypoxic-ischemic injury at P3 on subsequent cerebral development. Three groups of P3 Wistar rats were investigated: group I underwent right carotid ligation followed by 6% hypoxia for 30 min (HI), group 2 had carotid ligation only, and group 3 had no intervention. At P21, in the HI group, the right cortical area was reduced compared with controls (p < 0.01). There were no significant alterations in the size of the dorsal hippocampus, striatum, and thalamus. The cortical myelinated area was reduced in the HI animals compared with controls (p < 0.01). There was a corresponding loss of myelinated axons extending up into the cortex, with deep cortical neuronal and axonal architecture markedly disrupted. Glial fibrillary acidic protein immunohistology showed a reactive gliosis in the deep parietal cortex (p < 0.01). Moderate HI injury in the immature rat brain compromised cortical growth and led to a selective alteration of cortical myelinated axons with persistent gliosis. These alterations induced at P3 by unilateral HI share neuropathological similarities with the diffuse white matter lesions found in VLBW infants.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HI:
-
hypoxia-ischemia
- WM:
-
white matter
- PVL:
-
periventricular leukomalacia
- MBP:
-
myelin basic protein
- GFAP:
-
glial fibrillary acidic protein
References
Volpe JJ 1997 Overview: perinatal and neonatal brain injury. Ment Retard Dev Disabil Res Rev 3: 1–2
Dammann O, Leviton A 1997 The role of perinatal brain damage in developmental disabilities: an epidemiologic perspective. Ment Retard Dev Disabil Res Rev 3: 13–21
Volpe JJ 1997 Brain injury in the premature infant: neuropathology, clinical aspects and pathogenesis. Ment Retard Dev Disabil Res Rev 3: 1–12
Leviton A, Gilles F 1996 Ventriculomegaly, delayed myelination, white matter hypoplasia, and “periventricular” leukomalacia: how are they related?. Pediatr Neurol 15: 127–136
Inder TE, Huppi PS 2000 In vivo studies of brain development by magnetic resonance techniques. Ment Retard Dev Disabil Res Rev 6: 59–67
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ 1999 Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46: 755–760
Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ 2001 Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107: 455–460
Dammann O, Hagberg H, Leviton A 2001 Is periventricular leukomalacia an axonopathy as well as an oligopathy?. Pediatr Res 49: 453–457
Dammann O, Leviton A 1997 Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res 42: 1–8
Volpe JJ 2001 Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50: 553–562
Dobbing J, Sands J 1979 Comparative aspects of the brain growth spurt. Early Hum Dev 3: 79–83
Clancy B, Darlington RB, Finlay BL 2001 Translating developmental time across mammalian species. Neuroscience 105: 7–17
Dani JW, Armstrong DM, Benowitz LI 1991 Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neuroscience 40: 277–287
Honig LS, Herrmann K, Shatz CJ 1996 Developmental changes revealed by immunohistochemical markers in human cerebral cortex. Cereb Cortex 6: 794–806
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC 2001 Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci 21: 1302–1312
Sirimanne ES, Guan J, Williams CE, Gluckman PD 1994 Two models for determining the mechanisms of damage and repair after hypoxic-ischaemic injury in the developing rat brain. J Neurosci Methods 55: 7–14
Reddy K, Mallard CE, Guan J, Marks KA, Bennet L, Gunning MI, Gunn AJ, Gluckman PD, Williams CE 1998 Maturational change in the cortical response to hypoperfusion injury in the fetal sheep. Pediatr Res 43: 674–682
Follett PL, Rosenberg PA, Volpe JJ, Jensen FE 2000 NBQX attenuates excitotoxic injury in developing white matter. J Neurosci 20: 9235–9241
Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS 2001 Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int J Dev Neurosci 19: 197–208
Liu Y, Silverstein FS, Skoff R, Barks JD 2002 Hypoxic-ischemic oligodendroglial injury in neonatal rat brain. Pediatr Res 51: 25–33
Uehara H, Yoshioka H, Kawase S, Nagai H, Ohmae T, Hasegawa K, Sawada T 1999 A new model of white matter injury in neonatal rats with bilateral carotid artery occlusion. Brain Res 837: 213–220
Towfighi J, Mauger D, Vannucci RC, Vannucci SJ 1997 Influence of age on the cerebral lesions in an immature rat model of cerebral hypoxia-ischemia: a light microscopic study. Brain Res Dev Brain Res 100: 149–160
Rice JEd, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Rorke LB 1992 Anatomical features of the developing brain implicated in pathogenesis of hypoxic-ischemic injury. Brain Pathol 2: 211–221
Fedrizzi E, Inverno M, Bruzzone MG, Botteon G, Saletti V, Farinotti M 1996 MRI features of cerebral lesions and cognitive functions in preterm spastic diplegic children. Pediatr Neurol 15: 207–212
Towfighi J, Mauger D 1998 Temporal evolution of neuronal changes in cerebral hypoxia-ischemia in developing rats: a quantitative light microscopic study. Brain Res Dev Brain Res 109: 169–177
Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM 2002 Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22: 455–463
McConnell SK, Ghosh A, Shatz CJ 1989 Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science 245: 978–982
Volpe JJ 1996 Subplate neurons-missing link in brain injury of the premature infant?. Pediatrics 97: 112–113
Marin-Padilla M 1997 Developmental neuropathology and impact of perinatal brain damage. II: white matter lesions of the neocortex. J Neuropathol Exp Neurol 56: 219–235
Kostovic I, Lukinovic N, Judas M, Bogdanovic N, Mrzljak L, Zecevic N, Kubat M 1989 Structural basis of the developmental plasticity in the human cerebral cortex: the role of the transient subplate zone. Metab Brain Dis 4: 17–23
Tanaka H, Araki M, Masuzawa T 1992 Reaction of astrocytes in the gerbil hippocampus following transient ischemia: immunohistochemical observations with antibodies against glial fibrillary acidic protein, glutamine synthetase, and S-100 protein. Exp Neurol 116: 264–274
Burtrum D, Silverstein FS 1994 Hypoxic-ischemic brain injury stimulates glial fibrillary acidic protein mRNA and protein expression in neonatal rats. Exp Neurol 126: 112–118
Hirayama A, Okoshi Y, Hachiya Y, Ozawa Y, Ito M, Kida Y, Imai Y, Kohsaka S, Takashima S 2001 Early immunohistochemical detection of axonal damage and glial activation in extremely immature brains with periventricular leukomalacia. Clin Neuropathol 20: 87–91
Deguchi K, Oguchi K, Takashima S 1997 Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol 16: 296–300
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the Health Research Council of New Zealand and from New Zealand Lotteries Board.
Rights and permissions
About this article
Cite this article
Sizonenko, S., Sirimanne, E., Mayall, Y. et al. Selective Cortical Alteration after Hypoxic-Ischemic Injury in the Very Immature Rat Brain. Pediatr Res 54, 263–269 (2003). https://doi.org/10.1203/01.PDR.0000072517.01207.87
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000072517.01207.87
This article is cited by
-
Bumetanide Attenuates Cognitive Deficits and Brain Damage in Rats Subjected to Hypoxia–Ischemia at Two Time Points of the Early Postnatal Period
Neurotoxicity Research (2023)
-
Tissue Injury and Astrocytic Reaction, But Not Cognitive Deficits, Are Dependent on Hypoxia Duration in Very Immature Rats Undergoing Neonatal Hypoxia–Ischemia
Neurochemical Research (2019)
-
Disorganization of Oligodendrocyte Development in the Layer II/III of the Sensorimotor Cortex Causes Motor Coordination Dysfunction in a Model of White Matter Injury in Neonatal Rats
Neurochemical Research (2018)
-
Pregnancy swimming causes short- and long-term neuroprotection against hypoxia–ischemia in very immature rats
Pediatric Research (2017)
-
Prenatal and Early Postnatal Environmental Enrichment Reduce Acute Cell Death and Prevent Neurodevelopment and Memory Impairments in Rats Submitted to Neonatal Hypoxia Ischemia
Molecular Neurobiology (2017)