Abstract
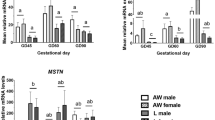
Uteroplacental insufficiency and subsequent intrauterine growth retardation (IUGR) increase the risk of insulin resistance in humans and rats. Aberrant skeletal muscle lipid metabolism contributes to the pathogenesis of insulin resistance. Peroxisome proliferator-activated receptor-γ co-activator-1 (PGC-1) is a transcriptional co-activator that affects gene expression of key lipid metabolizing enzymes such as carnitine palmitoyl-transferase I (mCPTI). Because gene expression of lipid metabolizing enzymes is altered in IUGR postnatal skeletal muscle, and we hypothesized that PGC-1 expression would be similarly affected. To prove this hypothesis, bilateral uterine artery ligation and sham surgery were used to produce IUGR and control rats respectively. Western Blotting demonstrated that PGC-1 hind limb skeletal muscle protein levels were increased in perinatal and postnatal IUGR rats. Conventional RT-PCR demonstrated that PGC-1 mRNA levels were similarly increased in perinatal hind limb skeletal muscle and juvenile extensor digitorum longus (EDL), but were decreased in juvenile soleus. Because a gender specific trend was noted in PGC-1 mRNA levels, real time RT-PCR was used for further differentiation. Real time RT-PCR revealed that changes in postnatal skeletal muscle PGC-1 expression were more marked in male IUGR rats versus female IUGR rats. Down stream targets of PGC-1 followed a similar pattern of expression. We conclude that PGC-1 expression is altered in rat IUGR skeletal muscle and speculate that it contributes to the pathogenesis of insulin resistance in the IUGR rat.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CD36:
-
fatty acid translocase
- CoxIV:
-
cytomchrome c oxidase subunit I
- EDL:
-
extensor digitorum longus
- IUGR:
-
intrauterine growth retardation
- mCPTI:
-
muscle isoform of carnitine palmitoyl-transferase I
- mTFA:
-
mitochondrial transcription factor A
- NRF-1:
-
nuclear respiratory factor 1
- PGC-1:
-
peroxisome proliferator-activated receptor-γ coactivator-1
- UCP-2:
-
uncoupling protein 2
REFERENCES
Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM 1993 Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 36: 62–67
Phillips DI, Barker DJ, Hales CN, Hirst S, Osmond C 1994 Thinness at birth and insulin resistance in adult life. Diabetologia 37: 150–154
Ogata ES, Bussey ME, LaBarbera A, Finley S 1985 Altered growth, hypoglycemia, hypoalaninemia, and ketonemia in the young rat: postnatal consequences of intrauterine growth retardation. Pediatr Res 19: 32–37
Sadiq HF, Das UG, Tracy TF, Devaskar SU 1999 Intra-uterine growth restriction differentially regulates perinatal brain and skeletal muscle glucose transporters. Brain Res 823: 96–103
Simmons RA, Templeton LJ, Gertz SJ 2001 Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50: 2279–2286
Vaag A, Alford F, Henriksen FL, Christopher M, Beck-Nielsen H 1995 Multiple defects of both hepatic and peripheral intracellular glucose processing contribute to the hyperglycaemia of NIDDM. Diabetologia 38: 326–336
Groop LC, Bonadonna RC, DelPrato S, Ratheiser K, Zyck K, Ferrannini E, DeFronzo RA 1989 Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J Clin Invest 84: 205–213
Lane RH, Chandorkar AK, Flozak AS, Simmons RA 1998 Intrauterine growth retardation alters mitochondrial gene expression and function in fetal and juvenile rat skeletal muscle. Pediatr Res 43: 563–570
Lane RH, Kelley DE, Ritov VH, Tsirka AE, Gruetzmacher EM 2001 Altered expression and function of mitochondrial beta-oxidation enzymes in juvenile intrauterine-growth-retarded rat skeletal muscle. Pediatr Res 50: 83–90
Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM 1999 Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124
Vega RB, Huss JM, Kelly DP 2000 The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor alpha in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20: 1868–1876
Lane RH, MacLennan NK, Hsu JL, Janke SM, Pham TD 2002 Increased hepatic peroxisome proliferator-activated receptor-gamma coactivator-1 gene expression in a rat model of intrauterine growth retardation and subsequent insulin resistance. Endocrinology 143: 2486–2490
Ek J, Andersen G, Urhammer SA, Gaede PH, Drivsholm T, Borch-Johnsen K, Hansen T, Pedersen O 2001 Mutation analysis of peroxisome proliferator-activated receptor-gamma coactivator-1 (PGC-1) and relationships of identified amino acid polymorphisms to Type II diabetes mellitus. Diabetologia 44: 2220–2226
Hara K, Tobe K, Okada T, Kadowaki H, Akanuma Y, Ito C, Kimura S, Kadowaki T 2002 A genetic variation in the PGC-1 gene and insulin resistance. Diabetologia 45: 740–743
Bussey ME, Finley S, LaBarbera A, Ogata ES 1985 Hypoglycemia in the newborn growth-retarded rat: delayed phosphoenolpyruvate carboxykinase induction despite increased glucagon availability. Pediatr Res 19: 363–367
Ogata ES, Swanson SL, Collins JW, Finley SL 1990 Intrauterine growth retardation: altered hepatic energy and redox states in the fetal rat. Pediatr Res 27: 56–63
Lane RH, Flozak AS, Ogata ES, Bell GI, Simmons RA 1996 Altered hepatic gene expression of enzymes involved in energy metabolism in the growth-retarded fetal rat. Pediatr Res 39: 390–394
Lane RH, Crawford SE, Flozak AS, Simmons RA 1999 Localization and quantification of glucose transporters in liver of growth-retarded fetal and neonatal rats. Am J Physiol 276: E135–E142
Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU 2001 Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol Regul Integr Comp Physiol 280: R183–R190
Tsirka AE, Gruetzmacher EM, Kelley DE, Ritov VH, Devaskar SU, Lane RH 2001 Myocardial gene expression of glucose transporter 1 and glucose transporter 4 in response to uteroplacental insufficiency in the rat. J Endocrinol 169: 373–380
Hegarty BD, Cooney GJ, Kraegen EW, Furler SM 2002 Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes 51: 1477–1484
Kloesz JL, Serdikoff CM, Maclennan NK, Adibi SA, Lane RH 2001 Uteroplacental insufficiency alters liver and skeletal muscle branched-chain amino acid metabolism in intrauterine growth-restricted fetal rats. Pediatr Res 50: 604–610
Wiedmer P, Klaus S, Ortmann S 2002 Energy metabolism of young rats after early postnatal overnutrition. Br J Nutr 88: 301–306
Talmadge RJ, Roy RR 1993 Electrophoretic separation of rat skeletal muscle myosin heavy-chain isoforms. J Appl Physiol 75: 2337–2340
LaFramboise WA, Daood MJ, Guthrie RD, Schiaffino S, Moretti P, Brozanski B, Ontell MP, Butler-Browne GS, Whalen RG, Ontell M 1991 Emergence of the mature myosin phenotype in the rat diaphragm muscle. Dev Biol 144: 1–15
Chomczynski P, Sacchi N 1987 Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159
Higuchi R, Fockler C, Dollinger G, Watson R 1993 Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Biotechnology N Y 11: 1026–1030
Menon RK, Shaufl A, Yu JH, Stephan DA, Friday RP 2001 Identification and characterization of a novel transcript of the murine growth hormone receptor gene exhibiting development- and tissue-specific expression. Mol Cell Endocrinol 172: 135–146
Ogata ES, Bussey ME, Finley S 1986 Altered gas exchange, limited glucose and branched chain amino acids, and hypoinsulinism retard fetal growth in the rat. Metabolism 35: 970–977
Economides DL, Nicolaides KH, Campbell S 1991 Metabolic and endocrine findings in appropriate and small for gestational age fetuses. J Perinat Med 19: 97–105
Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP 2000 Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest 106: 847–856
Tunstall RJ, Mehan KA, Wadley GD, Collier GR, Bonen A, Hargreaves M, Cameron-Smith D 2002 Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 283: E66–E72
Goto M, Terada S, Kato M, Katoh M, Yokozeki T, Tabata I, Shimokawa T 2000 cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 274: 350–354
Michael LF, Wu Z, Cheatham RB, Puigserver P, Adelmant G, Lehman JJ, Kelly DP, Spiegelman BM 2001 Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A 98: 3820–3825
Pin CL, Merrifield PA 208 1997 Regionalized expression of myosin isoforms in heterotypic myotubes formed from embryonic and fetal rat myoblasts in vitro. Dev Dyn 420–431
Ozaki K, Matsuura T, Narama I 2001 Histochemical and morphometrical analysis of skeletal muscle in spontaneous diabetic WBN/Kob rat. Acta Neuropathol (Berl) 102: 264–270
Larsson H, Daugaard JR, Kiens B, Richter EA, Ahren B 1999 Muscle fiber characteristics in postmenopausal women with normal or impaired glucose tolerance. Diabetes Care 22: 1330–1338
Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL 1995 Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol 268: E453–E457
Rubinstein NA, Kelly AM 1981 Development of muscle fiber specialization in the rat hindlimb. J Cell Biol 90: 128–144
Kugelberg E 1976 Adaptive transformation of rat soleus motor units during growth. J Neurol Sci 27: 269–289
Chessex P, Reichman B, Verellen G, Putet G, Smith JM, Heim T, Swyer PR 1984 Metabolic consequences of intrauterine growth retardation in very low birthweight infants. Pediatr Res 18: 709–713
Kelley DE, Simoneau JA 1994 Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94: 2349–2356
Walker M, Berrish TS, Stewart MW, Humphriss DB, Barriocanal L, Alberti KG 1997 Metabolic heterogeneity in impaired glucose tolerance. Metabolism 46: 914–917
Blaak EE, Wolffenbuttel BH, Saris WH, Pelsers MM, Wagenmakers AJ 2001 Weight reduction and the impaired plasma-derived free fatty acid oxidation in type 2 diabetic subjects. J Clin Endocrinol Metab 86: 1638–1644
He J, Watkins S, Kelley DE 2001 Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50: 817–823
Horton TJ, Gayles EC, Prach PA, Koppenhafer TA, Pagliassotti MJ 1997 Female rats do not develop sucrose-induced insulin resistance. Am J Physiol 272: R1571–R1576
Hevener A, Reichart D, Janez A, Olefsky J 2002 Female rats do not exhibit free fatty acid-induced insulin resistance. Diabetes 51: 1907–1912
Binnert C, Koistinen HA, Martin G, Andreelli F, Ebeling P, Koivisto VA, Laville M, Auwerx J, Vidal H 2000 Fatty acid transport protein-1 mRNA expression in skeletal muscle and in adipose tissue in humans. Am J Physiol Endocrinol Metab 279: E1072–E1079
Laws A, Hoen HM, Selby JV, Saad MF, Haffner SM, Howard BV 1997 Differences in insulin suppression of free fatty acid levels by gender and glucose tolerance status. Relation to plasma triglyceride and apolipoprotein B concentrations. Insulin Resistance Atherosclerosis Study (IRAS) Investigators. Arterioscler Thromb Vasc Biol 17: 64–71
Sumner AE, Kushner H, Sherif KD, Tulenko TN, Falkner B, Marsh JB 1999 Sex differences in African-Americans regarding sensitivity to insulin's glucoregulatory and antilipolytic actions. Diabetes Care 22: 71–77
Knutti D, Kaul A, Kralli A 2000 A tissue-specific coactivator of steroid receptors, identified in a functional genetic screen. Mol Cell Biol 20: 2411–2422
Pan DA, Lillioja S, Kriketos AD, Milner MR, Baur LA, Bogardus C, Jenkins AB, Storlien LH 1997 Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes 46: 983–988
Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L 1999 Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48: 1600–1606
Author information
Authors and Affiliations
Corresponding author
Additional information
National Institute of Child Health and Human Development Grant K08-BD-01225-1, R01 HD41075 and an American Diabetes Association Grant supported this work.
Rights and permissions
About this article
Cite this article
Lane, R., MacLennan, N., Daood, M. et al. IUGR Alters Postnatal Rat Skeletal Muscle Peroxisome Proliferator-Activated Receptor-γ Coactivator-1 Gene Expression in a Fiber Specific Manner. Pediatr Res 53, 994–1000 (2003). https://doi.org/10.1203/01.PDR.0000064583.40495.51
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000064583.40495.51
This article is cited by
-
Echocardiographic assessment of fetal cardiac function in the uterine artery ligation rat model of IUGR
Pediatric Research (2021)
-
Uteroplacental insufficiency down regulates insulin receptor and affects expression of key enzymes of long-chain fatty acid (LCFA) metabolism in skeletal muscle at birth
Cardiovascular Diabetology (2008)
-
Fatty acid oxidation in the human fetus: Implications for fetal and adult disease
Journal of Inherited Metabolic Disease (2006)