Abstract
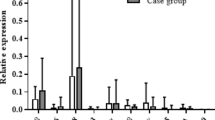
The mucosal immune system is overactivated in Crohn disease (CD) and viral infections have been associated with clinical exacerbations. To investigate the potential association between mucosal inflammation and the cytokines involved in the early response to viruses, we analyzed colonic tissue levels of IL-2Rα, interferon-α, and IL-15 in CD. Patients undergoing diagnostic colonoscopy were classified into controls (n = 22) and three CD groups based on the histologic severity of inflammation and clinical activity: a) severely active CD (n = 3); b) mild to moderately active CD (n = 14); and c) quiescent CD (n = 23). Rectal biopsies (two per patient) were homogenized and cytokine levels determined by ELISA kits. Statistical analysis was performed by ANOVA with Tukey and Scheffé tests. IL-2Rα levels were increased in the active CD group compared with the quiescent CD group: a) 405 ± 87, b) 159 ± 31, and c) 33 ± 15 pg/mg DNA (p < 0.001). The latter group was similar to controls (39 ± 20 pg/mg DNA). Furthermore, a linear correlation (r = 0.98) between IL-2Rα and disease activity (Van Hees index) was observed. IL-15 levels were also higher in active compared with quiescent CD and controls: a) 0.69 ± 0.23 and b) 0.72 ± 0.31 versus c) 0.28 ± 0.21 and 0.28 ± 0.14 pg/mg DNA for controls (p < 0.05). Interferon-α levels were undetectable in all samples. Our data suggest that IL-2Rα tissue levels correlate with CD activity. IL-15 is also overproduced in inflamed CD tissue. The lack of a parallel elevation of interferon-α does not support a role for viral induction of IL-15 in inflamed CD samples.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CD:
-
Crohn disease
- IL-2Rα:
-
IL-2 receptor α-chain
- IBD:
-
inflammatory bowel disease
- IFN-α:
-
interferon-α
References
Watts DA, Satsangi J 2002 The genetic jigsaw of inflammatory bowel disease. Gut 50: iii31–iii36
Fiocchi C 1998 Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115: 182–205
Duff GW 1989 Peptide regulatory factors in non-malignant disease. Lancet 1: 1432
Choy M, Walker-Smith J, Williams C, MacDonald T 1990 Differential expression of CD 25 (interleukin-2 receptor) on lamina propria T cells and macrophages in the intestinal lesions in Crohn's disease and ulcerative colitis. Gut 31: 1365–1370
Crabtree JE, Juby LD, Heatley RV, Lobo AJ, Bullimore DW, Axon ATR 1990 Soluble interleukin-2 receptor in Crohn's disease: relation of serum concentrations to disease activity. Gut 31: 1033–1036
Mahida YR, Gallagher A, Kurlak L, Hawkey CJ 1990 Plasma and tissue interleukin-2 receptor levels in inflammatory bowel disease. Clin Exp Immunol 82: 75–80
Mueller Ch, Knoflach P, Zielinski CC 1990 T-cell activation in Crohn's disease. Gastroenterology 98: 639–646
Williams A, Symons J, Watchet K, Duff GW 1992 Soluble interleukin-2 receptor and disease activity in Crohn's disease. J Autoimmun 5: 251–259
Schurmann G, Betzler M, Post S, Herfarth CH, Meuer S 1992 Soluble interleukin-2 receptor, interleukin-6 and interleukin-1β in patients with Crohn's disease and ulcerative colitis: preoperative levels and postoperative changes of serum concentrations. Digestion 51: 51–59
Kirman I, Nielsen OH, Kjaersgaard E, Brynskov J 1995 Interleukin-2 receptor α and β chain expression by circulating αβ and γδ T cells in inflammatory bowel disease. Dig Dis Sci 40: 291–295
Louis E, Belaiche J, Van Kemseke C, Schaaf N, Mahieu P, Mary JY 1995 Soluble interleukin-2 receptor in Crohn's disease. Assessment of disease activity and prediction of relapse. Dig Dis Sci 40: 1750–1756
Brynskov J, Tvede N, Andersen CB, Vilien M 1992 Increased concentrations of interleukin 1β, interleukin-2, and soluble interleukin-2 receptors in endoscopical mucosal biopsy specimens with active inflammatory bowel disease. Gut 33: 55–58
Matsuura T, West G, Youngman K, Klein J, Fiocchi C 1993 Immune activation genes in inflammatory bowel disease. Gastroenterology 104: 448–458
Fehniger T, Caligiuri M 2001 Interleukin 15: biology and relevance to human disease. Blood 97: 14–32
Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn MA, Ahdieh M, Johnson L, Alderson M, Watson J, Anderson D, Giri J 1994 Cloning of a T cell growth factor that interacts with the β chain of the interleukin-2 receptor. Science 264: 965–968
Carson WE, Ross ME, Baiocchi RA, Marien MJ, Boiani N, Grabstein K, Caligiuri MA 1995 Endogenous production of interleukin 15 by activated human monocytes is critical for optimal production of interferon-γ by natural killer cells in vitro. J Clin Invest 96: 2578–2582
Doherty TM, Seder RA, Sher A 1996 Induction and regulation of IL-15 expression in murine macrophages. J Immunol 156: 735–741
Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky K 1996 Intestinal epithelial cells both express and respond to interleukin 15. Gastroenterology 111: 1706–1713
Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA 1996 IL-15 a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity 4: 329–336
Kirman I, Nielsen OH 1996 Increased numbers of interleukin-15-expressing cells in active ulcerative colitis. Am J Gastroenterol 91: 1789–1794
Liu Z, Geboes K, Colpaert S, Haens G, Rutgeerts P, Ceuppens J 2000 IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol 164: 3608–3615
Sakai T, Kusugami K, Nishimura H, Ando T, Yamaguchi T, Ohsuga M, Ina K, Enomoto A, Kimura Y, Yoshikai Y 1998 Interleukin 15 activity in the rectal mucosa of inflammatory bowel disease. Gastroenterology 114: 1237–1243
Vainer B, Nielsen OH, Hendel J, Horn T, Kirman I 2000 Colonic expression and synthesis of interleukin 13 and interleukin 15 in inflammatory bowel disease. Cytokine 12: 1531–1536
Flamand L, Stefanescu I, Menezes J 1996 Human herpesvirus-6 enhances natural killer cell cytotoxicity via IL-15. J Clin Invest 97: 1373–1381
Fawaz L, Sharif-Askari E, Menezes J 1999 Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J Immunol 163: 4473–4480
Guidotti L, Chisari F 2001 Noncytolytic control of viral infections by the innate and adaptative immune response. Annu Rev Immunol 19: 65–91
Le Page C, Genin P, Baines MG, Hiscott J 2000 Interferon activation and innate immunity. Rev Immunogenet 2: 374–386
Waldman T, Tagaya Y 1999 The multifaceted regulation of IL-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol 17: 19–49
Balfour S 1995 Microbial agents in the pathogenesis, differential diagnosis, and complications of inflammatory bowel diseases. In: Blaser M, Smith P, Ravdin J, Greenberg H, Guerrant R (eds) Infections of the Gastrointestinal Tract. Raven Press Ltd, New York, pp 435–458.
Cottone M, Pietrosi G, Martorana G, Casa A, Pecoraro G, Oliva L, Orlando A, Rosselli M, Rizzo A, Pagliaro L 2001 Prevalence of cytomegalovirus infection in severe refractory ulcerative and Crohn's colitis. Am J Gastroenterol 96: 773–775
Wakefield A, Fox J, Sawyerr A, Taylor JE, Sweenie CH, Smith M, Emery VC, Hudson M, Tedder RS, Pounder RE 1992 Detection of herpesvirus DNA in the large intestine of patients with ulcerative colitis and Crohn's disease using the nested polymerase chain reaction. J Med Virol 38: 183–190
Spieker T, Herbst H 2000 Distribution and phenotype of Epstein-Bar virus-infected cells in inflammatory bowel disease. Am J Pathol 157: 51–57
Yanai H, Shimizu N, Nagasaki S, Mitani N, Okita K 1999 Epstein-Barr virus infection of the colon with inflammatory bowel disease. Am J Gastroenterology 94: 1582–1586
Wakefield A, Pittilo R, Sim R, Cosby SL, Stephenson JR, Dhillon AP, Pounder RE 1993 Evidence of persistent measles virus infection in Crohn's disease. J Med Virol 39: 345–353
Haga Y, Funakoshi O, Kuroe K, Kanazawa K, Nakajima H, Saito H, Murata Y, Munakata A, Yoshida Y 1996 Absence of measles viral genomic sequence in intestinal tissue from Crohn's disease by nested polymerase chain reaction. Gut 38: 211–215
Harvey RF, Bradshaw JM 1980 A simple index of Crohn's disease activity. Lancet 1: 514
Van Hees P, Van Elteren PH, Van Lier HJJ, Van Tongeren JH 1980 An index of inflammatory activity in patients with Crohn's disease. Gut 21: 279–286
Riddell RH 1997 Histopathology of ulcerative colitis. In: Allan R, Rhodes J, Hanauer S, Keighley M, Alexander-Williams J, Fazio V (eds) Inflammatory Bowel Disease, 3rd ed. Churchill-Livingstone, Edinburgh, pp 291–309.
Goldblum J, Petras R 1997 Histopathology of Crohn's disease. In: Allan R, Rhodes J, Hanauer S, Keighley M, Alexander-Williams J, Fazio V (eds) Inflammatory Bowel Disease, 3rd ed. Churchill-Livingstone, Edinburgh, pp 311–316.
Eliakim R, Rachmilewitz D 1997 Assessing disease activity in Crohn's disease—are we there yet?. Eur J Gastroenterol Hepatol 9: 929–930
Modigliani R, Mary J, Simon J, Cortot A, Soule JC, Gendre JP, Rene E 1990 Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Gastroenterology 98: 811–818
Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M 1990 Predictability of the postoperative course of Crohn's disease. Gastroenterology 99: 956–963
Otley A, Loonen H, Parekh N, Corey M, Sherman P, Griffiths A 1999 Assessing activity of pediatric Crohn's disease: which index to use?. Gastroenterology 116: 527–531
Waldmann T, Tagaya Y, Bamford R 1998 Interleukin-2, interleukin-15 and their receptors. Intern Rev Immunol 16: 205–226
Reimund J, Wttersheim C, Dumont S, Muller C, Baumann R, Poindron P, Duclos B 1996 Mucosal inflammatory cytokine production by intestinal biopsies in patients with ulcerative colitis and Crohn's disease. J Clin Immunol 16: 144–150
Dionne S, D Agata D, Hiscott J, Vanounou T, Seidman E 1998 Colonic explant production of IL-1 and its receptor antagonist is imbalanced in inflammatory bowel disease. Clin Exp Immunol 112: 435–442
Dionne S, Ruemmele F, Laberge S, Seidman E 2000 The effect of inflammation severity and of treatment on the production and release of TNFα by colonic explants in inflammatory bowel disease. Aliment Pharmacol Ther 14: 1435–1442
Masuyama T, Matsuo M, Ichimaru T, Ishii K, Tsuchiya K, Hamasaki Y 2002 Possible contribution of interferon-alpha to febrile seizures in influenza. Pediatr Neurol 27: 289–292
Acknowledgements
The authors thank the members of the Pediatric Gastroenterology Service for their assistance in the recruitment of patients and providing biopsy specimens.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by a research grant from the Crohn's and Colitis Foundation of Canada (E.G.S., J.M.), by an IBD Research Chair Award from the CIHR/CCFC (E.G.S.), and by Research Fellowship Awards from the Saint-Justine Hospital Research Foundation and the Canadian Association of Gastroenterology/Axcan Pharma (M.S.).
Rights and permissions
About this article
Cite this article
Silva, M., Menezes, J., Wizman, S. et al. Cytokine Tissue Levels as Markers of Disease Activity in Pediatric Crohn Disease. Pediatr Res 54, 456–461 (2003). https://doi.org/10.1203/01.PDR.0000083002.91602.40
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000083002.91602.40
This article is cited by
-
Disease activity and mucosal healing in inflammatory bowel disease: a new role for histopathology?
Virchows Archiv (2018)
-
Dendritic Cells and Toll-Like Receptors 2 and 4 in the Ileum of Crohn's Disease Patients
Digestive Diseases and Sciences (2008)
-
Functional correlates of the interleukin-1 receptor antagonist gene polymorphism in the colonic mucosa in ulcerative colitis
Genes & Immunity (2004)