Abstract
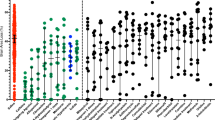
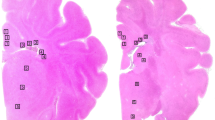
The hypothesis was tested that treatment with allopurinol, a xanthine oxidase inhibitor, or deferoxamine, a chelator of nonprotein-bound iron, preserved cerebral energy metabolism, attenuated development of edema, and improved histologic outcome in the newborn piglet at 24 h after hypoxia-ischemia. Thirty-two newborn piglets were subjected to 1 h of hypoxia-ischemia by occluding both carotid arteries and reducing the fraction of inspired oxygen; five newborn piglets served as sham-operated controls. The depth of hypoxia-ischemia was controlled by phosphorous magnetic resonance spectroscopy. Upon reperfusion and reoxygenation, piglets received vehicle (n = 12), allopurinol (30 mg/kg/d, n = 10), or deferoxamine (12.5 mg/kg/d, n = 10). The cerebral energy status was determined with phosphorous magnetic resonance spectroscopy. The presence of vasogenic edema was assessed by T2-weighted magnetic resonance imaging. Brain cell injury was assessed with caspase-3 activity, histology, and terminal deoxynucleotidyl transferase-mediated dUTP-biotin in situ nick end (TUNEL)-labeling. At 24 h after hypoxia-ischemia, the phosphocreatine/inorganic phosphate ratios were significantly decreased in vehicle-treated, but not in allopurinol- or deferoxamine-treated piglets. Water T2 values were significantly increased at 24 h after hypoxia-ischemia in cerebral cortex, thalamus, and striatum of vehicle-treated piglets, but not in allopurinol- and deferoxamine-treated piglets. No differences in caspase-3 activity, histologic outcome, or TUNEL-labeling were demonstrated between the three treatment groups. We suggest that allopurinol and deferoxamine may have an additional value in the treatment of perinatal hypoxia-ischemia with other neuroprotective agents or in combination with hypothermia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BE:
-
base excess
- Fio2:
-
fraction of inspired oxygen
- NPBI:
-
nonprotein-bound iron
- MABP:
-
mean arterial blood pressure
- MRI:
-
magnetic resonance imaging
- PCr:
-
phosphocreatine
- Pi:
-
inorganic phosphate
- 31P-MRS:
-
phosphorous magnetic resonance spectroscopy
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP-biotin in situ nick end labeling
References
Ferriero DM 2001 Oxidant mechanisms in neonatal hypoxia-ischemia. Dev Neurosci 23: 198–202
Delivoria-Papadopoulos M, Mishra OP 1998 Mechanisms of cerebral injury in perinatal asphyxia and strategies for prevention. J Pediatr 132: S30–S34
Fellman V, Raivio KO 1997 Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 41: 599–606
Halliwell B 1989 Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action?. Free Radic Biol Med 7: 645–651
Gluckman PD, Williams CE 1992 When and why do brain cells die?. Dev Med Child Neurol 34: 1010–1014
Lorek A, Takei Y, Cady EB, Wyatt JS, Penrice J, Edwards AD, Peebles D, Wylezinska M, Owen Reece H, Kirkbride V, et al 1994 Delayed (“secondary”) cerebral energy failure after acute hypoxia-ischemia in the newborn piglet: continuous 48-hour studies by phosphorus magnetic resonance spectroscopy. Pediatr Res 36: 699–706
Shadid M, Moison R, Steendijk P, Hiltermann L, Berger HM, Van Bel F 1998 The effect of antioxidative combination therapy on post hypoxic-ischemic perfusion, metabolism, and electrical activity of the newborn brain. Pediatr Res 44: 119–124
Van Bel F, Shadid M, Moison RMW, Dorrepaal CA, Fontijn J, Monteiro L, Van de Bor M, Berger HM 1998 Effect of allopurinol on postasphyxial free radical formation, cerebral hemodynamics, and electrical brain activity. Pediatrics 101: 185–193
Marro PJ, McGowan JE, Razdan B, Mishra OP, Delivoria-Papadopoulos M 1994 Effect of allopurinol on uric acid levels and brain cell membrane Na+,K(+)-ATPase activity during hypoxia in newborn piglets. Brain Res 650: 9–15
Palmer C, Towfighi J, Roberts RL, Heitjan DF 1993 Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res 33: 405–411
Hurn PD, Koehler RC, Blizzard KK, Traystman RJ 1995 Deferoxamine reduces early metabolic failure associated with severe cerebral ischemic acidosis in dogs. Stroke 26: 688–694
Peeters-Scholte C, Koster JG, Veldhuis W, van den Tweel E, Zhu C, Kops N, Blomgren K, Bär D, van Buul-Offers SC, Hagberg H, Nicolay K, Van Bel F, Groenendaal F 2002 Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke 33: 2304–2310
Naressi A, Couturier C, Devos JM, Janssen M, Mangeat C, de Beer R, Graveron-Demilly D 2001 Java-based graphical user interface for the MRUI quantitation package. MAGMA 12: 141–152
Wung WE, Howell SB 1980 Simultaneous liquid chromatography of 5-fluorouracil, uridine, hypoxanthine, xanthine, uric acid, allopurinol, and oxipurinol in plasma. Clin Chem 26: 1704–1708
Whitaker JR, Granum PE 1980 An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal Biochem 109: 156–159
Kime R, Gibson A, Yong W, Hider R, Powers H 1996 Chromatographic method for the determination of non-transferrin-bound iron suitable for use on the plasma and bronchoalveolar lavage fluid of preterm babies. Clin Sci Lond 91: 633–638
Felix B, Leger ME, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP 1999 Stereotaxic atlas of the pig brain. Brain Res Bull 49: 1–137
Peeters-Scholte C, van den Tweel E, Ioroi T, Post I, Braun K, Veldhuis W, Nicolay K, Groenendaal F, Van Bel F 2002 Pharmacological interventions in the newborn piglet in the first 24h after hypoxia-ischaemia: a haemodynamic and electrophysiological perspective. Exp Brain Res 147: 200–208
Williams GD, Palmer C, Heitjan DF, Smith MB 1992 Allopurinol preserves cerebral energy metabolism during perinatal hypoxia-ischemia: a 31P NMR study in unanesthetized immature rats. Neurosci Lett 144: 103–106
Palmer C, Vannucci RC, Towfighi J 1990 Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res 27: 332–336
Betz AL 1985 Identification of hypoxanthine transport and xanthine oxidase activity in brain capillaries. J Neurochem 44: 574–579
Phillis JW, Sen S 1993 Oxypurinol attenuates hydroxyl radical production during ischemia/reperfusion injury of the rat cerebral cortex: an ESR study. Brain Res 628: 309–312
Moorhouse PC, Grootveld M, Halliwell B, Quinlan JG, Gutteridge JM 1987 Allopurinol and oxypurinol are hydroxyl radical scavengers. FEBS Lett 213: 23–28
Hudome S, Palmer C, Roberts RL, Mauger D, Housman C, Towfighi J 1997 The role of neutrophils in the production of hypoxic-ischemic brain injury in the neonatal rat. Pediatr Res 41: 607–616
Ko KM, Godin DV 1990 Inhibition of transition metal ion-catalysed ascorbate oxidation and lipid peroxidation by allopurinol and oxypurinol. Biochem Pharmacol 40: 803–809
Peterson DA, Kelly B, Gerrard JM 1986 Allopurinol can act as an electron transfer agent. Is this relevant during reperfusion injury?. Biochem Biophys Res Commun 137: 76–79
Lebon V, Petersen KF, Cline GW, Shen J, Mason GF, Dufour S, Behar KL, Shulman GI, Rothman DL 2002 Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci 22: 1523–1531
Palmer C, Roberts RL, Bero C 1994 Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke 25: 1039–1045
Feng Y, LeBlanc MH, LeBlanc EB, Parker CC, Fratkin JD, Qian XB, Patel DM, Huang M, Smith EE, Vig PJ 2000 Desmethyl tirilazad improves neurologic function after hypoxic ischemic brain injury in piglets. Crit Care Med 28: 1431–1438
Cable H, Lloyd JB 1999 Cellular uptake and release of two contrasting iron chelators. J Pharm Pharmacol 51: 131–134
Shadid M, Buonocore G, Groenendaal F, Moison R, Ferrali M, Berger HM, Van Bel F 1998 Effect of deferoxamine and allopurinol on non-protein-bound iron concentrations in plasma and cortical brain tissue of newborn lambs following hypoxia-ischemia. Neurosci Lett 248: 5–8
deLemos RA, Roberts RJ, Coalson JJ, deLemos JA, Null DM, Gerstmann DR 1990 Toxic effects associated with the administration of deferoxamine in the premature baboon with hyaline membrane disease. Am J Dis Child 144: 915–919
Varani J, Dame MK, Diaz M, Stoolman L 1996 Deferoxamine interferes with adhesive functions of activated human neutrophils. Shock 5: 395–401
Groenendaal F, Shadid M, McGowan JE, Mishra OP, Van Bel F 2000 Effects of deferoxamine, a chelator of free iron, on Na+,K+-ATPase activity of cortical brain cell membrane during early reperfusion after hypoxia-ischemia in newborn lambs. Pediatr Res 48: 560–564
Dorrepaal CA, Berger HM, Benders MJ, Zoeren-Grobben D, Van deBor M, Van Bel F 1996 Nonprotein-bound iron in postasphyxial reperfusion injury of the newborn. Pediatrics 98: 883–889
Weber E, Allegrini PR, Sauer D 1993 Assessment of infarct volume in the mouse brain: correlation of magnetic resonance imaging with morphometry. In Vivo 7: 335–338
Acknowledgements
The authors thank Evelyn van den Tweel, Tomoaki Ioroi, and Nicole Hamers for their help with the experiments and the histology. We also thank the biotechnicians of the Central Laboratory Animal Institute of the Utrecht University for their enthusiastic help during the experiments; Robin de Graaf for designing the MR sequences; Changlian Zhu, from the Perinatal Center in Göteborg, Sweden, for assisting in the caspase-3 measurements; Tessa Ververs, of the pharmacological department of the University Medical Center, Utrecht, the Netherlands, for the determination of the allopurinol and oxypurinol concentrations; Cheraar Leusink for technical support during the animal experiments; and Gerard van Vliet for constructing the MR head coils. The MRVI software package was kindly provided by the participants of the EU Network programmes: Human Capital and Mobility, CHRX-CT94-0432 and Training and Mobility of Researchers, ERB-FMRX-CT970160.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the Netherlands Organization for Scientific Research (NWO-AGIKO stipend 920–03–039) and the University Medical Center Utrecht (“Sterproject”). Oxford Instruments provided a cerebral function monitor, and Tyco Healthcare provided a Nellcor NPB-290 pulse oximeter.
Rights and permissions
About this article
Cite this article
Peeters-Scholte, C., Braun, K., Koster, J. et al. Effects of Allopurinol and Deferoxamine on Reperfusion Injury of the Brain in Newborn Piglets after Neonatal Hypoxia-Ischemia. Pediatr Res 54, 516–522 (2003). https://doi.org/10.1203/01.PDR.0000081297.53793.C6
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000081297.53793.C6
This article is cited by
-
New possibilities for neuroprotection in neonatal hypoxic-ischemic encephalopathy
European Journal of Pediatrics (2022)
-
Critical Evaluation of the Changes in Glutamine Synthetase Activity in Models of Cerebral Stroke
Neurochemical Research (2015)
-
Evaluation effects of allopurinol and FSH on reduction of ischemia–reperfusion injury and on preservation of follicle after heterotopic auto‐transplantation of ovarian tissue in mouse
Reproductive Medicine and Biology (2014)
-
Antenatal allopurinol for reduction of birth asphyxia induced brain damage (ALLO-Trial); a randomized double blind placebo controlled multicenter study
BMC Pregnancy and Childbirth (2010)