Abstract
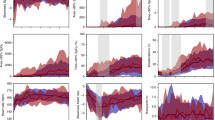
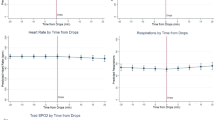
The aim of this study was to investigate whether the mean around which arterial oxygen fluctuations take place was important in a unique animal model of oxygen-induced retinopathy. Retinopathy of prematurity (ROP) is associated with fluctuating arterial oxygen. A recent retrospective study suggested that management of high-risk preterm infants at lower oxygen saturations was associated with less severe ROP. Rat pups were raised in a variable oxygen environment around a high (24%), normal (21%) or low (17%) mean inspired oxygen for 14 d. Rat pups raised in the high (24%) mean variable oxygen environment had more retarded retinal vascular development than did rats raised in an environment that fluctuated around 21% mean oxygen. In contrast, rats raised in a lower mean (17%) but still variable oxygen environment had no discernible retinal differences from controls raised in constant room air. Rats raised in a relatively hypoxic but variable oxygen environment develop less severe retinal vascular abnormalities than those raised in variable oxygen around higher oxygen means.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- C:
-
group raised in room air
- DAB:
-
diaminobenzidine
- Hi:
-
variable oxygen profile around 24% oxygen mean
- IQR:
-
interquartile range
- Lo:
-
variable oxygen profile around 17% oxygen mean
- N:
-
variable oxygen profile around 21% oxygen mean
- PFA:
-
paraformaldehyde
- ROP:
-
retinopathy of prematurity
- TBS:
-
Tris-buffered saline
- VEGF:
-
vascular endothelial growth factor
References
Fleck BW, Dangata Y 1994 Causes of visual handicap in the Royal Blind School, Edinburgh, 1991-2. Br J Ophthalmol 78: 421
Gilbert C, Rahi J, Eckstein M, O'Sullivan J, Foster A 1997 Retinopathy of prematurity in middle-income countries. Lancet 350: 12–14
Campbell K 1951 Intensive oxygen therapy as a possible cause of retrolental fibroplasia. A clinical approach. Med J Aust 2: 48
Ashton N, Ward B, Serpell G 1954 Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol 38: 397–430
Patz A, Eastham A, Higginbotham DH, Kleh T 1953 Oxygen studies in retrolental fibroplasia. Am J Ophthalmol 36: 1511–1522
Patz A 1982 The role of oxygen in retrolental fibroplasia. Am J Ophthalmol 94: 715–743
McColm JR, Fleck BW 2001 Retinopathy of prematurity—causation. Semin Neonatol 6: 453–460
Cunningham S, Fleck BW, Elton RA, McIntosh N 1995 Transcutaneous oxygen levels in retinopathy of prematurity. Lancet 346: 1464–1465
Saito Y, Omoto T, Cho Y, Hatsukawa Y, Fujimura M, Takeuchi T 1993 The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefes Arch Clin Exp Ophthalmol 231: 151–156
Reynaud X, Dorey KC 1994 Extraretinal neovascularization induced by hypoxic episodes in the neonatal rat. Invest Ophthalmol Vis Sci 35: 3169–3177
Penn JS, Henry MM, Wall PT, Tolman BL 1995 The range of PaO2 variation determines the severity of oxygen induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci 36: 2063–2070
Cunningham S, McColm JR, Wade J, Sedowofia K, McIntosh N, Fleck BW 2000 A novel model of retinopathy of prematurity simulating preterm oxygen variability in the rat. Invest Ophthalmol Vis Sci 41: 4275–4280
Mok JYQ, Hak H, McLaughin FJ, Pintar M, Canny GJ, Levison H 1988 Effect of age and state of wakefulness on transcutaneous oxygen values in preterm infants: a longitudinal study. J Pediatr 113: 706–709
Tin W, Milligan DWA, Pennefather PM, Hey E 2001 Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonat Ed 84: 106–110
Anderson CG, Benitz WE, Madan A 2002 Retinopathy of prematurity (ROP) and pulse oximetry: a national survey of recent practices. Pediatr Res 51: 367A(abstr)
Stone J, Itin A, Alon T, Peer J, Gnessin H, Chan-Ling T, Keshet E 1995 Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci 15: 4738–4747
McLeod DS, Brownstein R, Lutty GA 1996 Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci 37: 300–311
Smith LEH, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA 1994 Oxygen induced retinopathy in the mouse. Invest Ophthalmol Vis Sci 35: 101–111
McColm JR, Cunningham S 2000 The development of a computer controlled system to simulate in rats, the rapid, frequent changes in oxygen experienced by preterm infants developing retinopathy of prematurity. J Med Eng Technol 24: 45–52
Cunningham S, Deere S, Elton RA, McIntosh N 1992 Neonatal physiological trend monitoring by computer. Int J Clin Monit Comput 9: 221–227
Fetus and Newborn Committee of the AAP 1992 Clinical considerations in the use of oxygen. In: Freeman RK, Poland RL, HEDIT JC, Merenstein GB (eds) Guidelines for Perinatal Care. American Colleges of Pediatrics/Obstetricians/Gynecologists, Elk Grove Village, IL
Holmes JM, Zhang S, Leske DA, Lanier WL 1998 Carbon-dioxide induced retinopathy in the neonatal rat. Curr Eye Res 17: 608–616
Chan-Ling T 1997 Glial, vascular and neuronal cytogenesis in whole-mounted cat retina. Microsc Res Tech 36: 1–16
Ricci B, Minicucci G, Manfredi A, Santo A 1995 Oxygen-induced retinopathy in the newborn rat: effects of hyperbarism and topical administration of timolol maleate. Graefes Arch Clin Exper Ophthalmol 233: 226–230
Roberto KA, Tolman BL, Penn JS 1996 Long-term retinal vascular abnormalities in an animal model of retinopathy of prematurity. Curr Eye Res 15: 932–937
Chan-Ling T, Gock B, Stone J 1995 The effect of oxygen on vasoformative cell division: evidence that ‘physiological hypoxia' is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci 36: 1201–1214
Risau W 1997 Mechanisms of angiogenesis. Nature 386: 671–674
Carmeliet P 2000 Mechanisms of angiogenesis and arteriogenesis. Nat Med 6: 389–395
Holmes JM, Duffner LA 1996 The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res 15: 403–409
The STOP-ROP Multicenter Study Group 2000 Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics 105: 295–310
Phelps DL 1988 Reduced severity of oxygen-induced retinopathy in kittens recovered in 28% oxygen. Pediatr Res 24: 106–109
Chan-Ling T, Gock B, Stone J 1995 Supplemental oxygen therapy: basis for noninvasive treatment of retinopathy of prematurity. Invest Ophthalmol Vis Sci 36: 1215–1230
McGregor ML, Bremer DL, Cole C, McClead RE, Phelps DL, Fellows RR, Oden N 2002 Retinopathy of prematurity outcome in infants with prethreshold retinopathy of prematurity and oxygen saturation >94% in room air: the high oxygen percentage in retinopathy of prematurity study. Pediatrics 110: 540–544
Chow LC, Wright KW, Sola A 2003 Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants?. Pediatrics 111: 339–345
Mortola JP, Lauzon A-M 1990 Body growth, lung and heart weight, and DNA content in newborn rats exposed to different levels of chronic hypoxia. Can J Physiol Pharmacol 68: 1590–1594
Ment LR, Schwartz M, Makuch RW, Stewart WB 1998 Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res 111: 197–203
Keith IM, Tjen AL, Kraiczi H, Ekman R 2000 Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol 279: H1571–H1578
Tin W 2002 Oxygen therapy: 50 years of uncertainty. Pediatrics 110: 615–616
Acknowledgements
The authors thank Dr. Rob A. Elton for assistance with the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the Royal Blind School Edinburgh, United Kingdom; Mason Medical Foundation Brighton, United Kingdom; Ross Foundation for the Prevention of Blindness Edinburgh, United Kingdom; Research into Eye Disease Trust, London, United Kingdom.
Rights and permissions
About this article
Cite this article
McColm, J., Cunningham, S., Wade, J. et al. Hypoxic Oxygen Fluctuations Produce Less Severe Retinopathy than Hyperoxic Fluctuations in a Rat Model of Retinopathy of Prematurity. Pediatr Res 55, 107–113 (2004). https://doi.org/10.1203/01.PDR.0000099772.66376.02
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000099772.66376.02
This article is cited by
-
The effects of oxygen stresses on the development of features of severe retinopathy of prematurity: knowledge from the 50/10 OIR model
Documenta Ophthalmologica (2010)
-
The development of the rat model of retinopathy of prematurity
Documenta Ophthalmologica (2010)
-
Pathogenese der Retinopathia praematurorum
Der Ophthalmologe (2008)
-
Cumulative illness severity and progression from moderate to severe retinopathy of prematurity
Journal of Perinatology (2007)
-
Oxygen and retinopathy of prematurity
Journal of Perinatology (2006)