Abstract
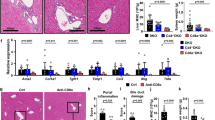
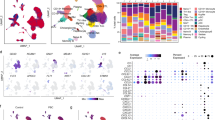
A proposed mechanism in the pathogenesis of biliary atresia involves an initial virus-induced, progressive T cell–mediated inflammatory obliteration of bile ducts. The aim of this study was to characterize the inflammatory environment present within the liver of infants with biliary atresia to gain insight into the role of a primary immune-mediated process versus a nonspecific secondary response to biliary obstruction. Frozen liver tissue obtained from patients with biliary atresia, neonatal giant cell hepatitis, total parenteral nutrition (TPN)–related cholestasis, choledochal cysts, and normal control subjects was used for fluorescent immunohistochemistry studies of cellular infiltrates, cytokine mRNA expression, and in situ hybridization for localization of cytokine-producing cells. Immunohistochemistry revealed increases in CD8+ and CD4+ T cells and Kupffer cells (CD68+) in the portal tracts of biliary atresia. Reverse transcription–PCR analysis of biliary atresia tissue showed a Th1-type cytokine profile with expression of IL-2, interferon-γ, tumor necrosis factor-α, and IL-12. This profile was not seen in normal, neonatal hepatitis or choledochal cyst livers but was present in TPN-related cholestasis. In situ hybridization revealed that the Th1 cytokine–producing cells were located in the portal tracts in biliary atresia and in the parenchyma of TPN-related cholestasis. A distinctive portal tract inflammatory environment is present in biliary atresia, involving CD4+ Th1 cell–mediated immunity. The absence of similar inflammation in other pediatric cholestatic conditions suggests that the portal tract inflammation in biliary atresia is not a secondary response to cholestasis but rather indicates a specific immune response involved in the pathogenesis of biliary atresia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CDC:
-
choledochal cyst
- HRP:
-
horseradish peroxidase
- IFN:
-
interferon
- NH:
-
neonatal giant cell hepatitis
- NK:
-
natural killer
- TNF:
-
tumor necrosis factor
- TPN:
-
total parenteral nutrition
References
Stein JE, Vacanti JP 1994 Biliary atresia and other disorders of the extrahepatic biliary tree. In: Suchy FJ (ed) Liver Disease in Children. Mosby, London, pp 426–442.
Tan CEL, Driver M, Howard ER, Moscoso GJ 1994 Extrahepatic biliary atresia: a first trimester event? Clues from light microscopy and immunohistochemistry. J Pediatr Surg 29: 808–814.
Horwitz MS, Sarvetnick N 1999 Viruses, host responses and autoimmunity. Immunol Rev 169: 241–253.
Sokol RJ, Mack CL 2001 Etiopathogenesis of biliary atresia. Semin Liver Dis 21: 517–524.
Schreiber RA, Kleinman RE 1993 Genetics, immunology and biliary atresia: an opening or a diversion?. J Pediatr Gastroenterol Nutr 16: 111–113.
Morecki R, Glaser JH, Cho S, Balistreri WF, Horwitz MS 1982 Biliary atresia and reovirus type 3 infection. N Engl J Med 307: 481–484.
Morecki R, Glaser JH, Johnson AB, Kress Y 1984 Detection of reovirus type 3 in the porta hepatis of an infant with extrahepatic biliary atresia: ultrastructural and immunocytochemical study. Hepatology 4: 1137–1142.
Tyler KL, Sokol RJ, Oberhaus SM, Le M, Karrer FM, Narkewicz MR, Tyson RW, Murphy JR, Low R, Brown WR 1998 Detection of reovirus RNA in hepatobiliary tissues from patients with extrahepatic biliary atresia and choledochal cysts. Hepatology 27: 1475–1482.
Brown WR, Sokol RJ, Levin MJ, Silverman A, Tamaru T, Lilly JR, Hall RJ, Cheney M 1998 Lack of correlation between infection with reovirus 3 and extrahepatic biliary atresia or neonatal hepatitis. J Pediatr 113: 670–676.
Steele MI, Marshall CM, Lloyd RE, Randolph VE 1995 Reovirus 3 not detected by reverse transcriptase mediated polymerase chain reaction analysis of preserved tissue from infants with cholestatic liver disease. Hepatology 21: 697–702.
Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, Uhnoo I, Greenberg SJ, Schakel K, Zhaori G, Fitzgerald J, Chong S, el-Yousef M, Nemeth A, Brown M, Piccoli D, Hyams J, Ruffin D, Rossi T 1996 Detection of group C rotavirus in infants with extrahepatic biliary atresia. J Infect Dis 174: 8–15.
Bobo L, Ojeh C, Chiu D, Machado A, Colombani P, Schwarz K 1997 Lack of evidence for rotavirus by polymerase chain reaction/enzyme immunoassay of hepatobiliary samples from children with biliary atresia. Pediatr Res 41: 229–234.
Fischler B, Ehrnst A, Forsgren M, Orvell C, Nemeth A 1998 The viral association of neonatal cholestasis in Sweden: a possible link between cytomegalovirus infection and extrahepatic biliary atresia. J Pediatr Gastroenterol Nutr 27: 57–64.
Jevon GP, Dimmic JE 1999 Biliary atresia and cytomegalovirus infection: a DNA study. Pediatr Dev Pathol 2: 11–14.
Bill AH, Haas JE, Foster GL 1977 Biliary atresia: histopathologic observations and reflections upon its natural history. J Pediatr Surg 12: 977–982.
Haas JE 1978 Bile duct and liver pathology in biliary atresia. World J Surg 2: 561–569.
Kobayashi H, Puri P, O'Brian DS, Surana R, Miyano T 1997 Hepatic overexpression of MHC class II antigens and macrophage-associated antigens (CD68) in patients with biliary atresia of poor prognosis. J Pediatr Surg 32: 590–593.
Urushihara N, Iwagaki H, Yagi T, Kohka H, Kobashi K, Morimoto Y, Yoshino T, Tanimoto T, Kurimoto M, Tanaka N 2000 Elevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresia. J Pediatr Surg 35: 446–449.
Tracy TF, Dillon P, Fox ES, Minnick K, Vogler C 1996 The inflammatory response in pediatric biliary disease: macrophage phenotype and distribution. J Pediatr Surg 31: 121–125.
Broome U, Nemeth A, Hultcrantz R, Scheynius A 1997 Different expression of HLA-DR and ICAM-1 in livers from patients with biliary atresia and Byler's disease. J Hepatol 26: 857–62.
Davenport M, Gonde C, Redkar R, Koukoulis G, Tredger M, Mieli-Vergani G, Portmann B, Howard ER 2001 Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Pediatr Surg 36: 1017–1025.
Eagar TN, Tompkins SM, Miller SD 2001 Helper T-cell subsets and control of the inflammatory response. In: Rich RR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HW Jr (eds) Clinical Immunology Principles and Practice. Mosby, London, pp 16.1–16.12
Ahmed AF, Ohtani H, Nio M, Funaki N, Shimaoda S, Nagura H, Ohi R 2001 CD8+ T cells infiltrating into bile ducts in biliary atresia do not appear to function as cytotoxic T cells: a clinicopathological analysis. J Pathol 193: 383–389.
Desmet VJ 2001 The cholangiopathies. In: Suchy FJ, Sokol RJ, Balistreri WF (eds) Liver Disease in Children. Lippincott Williams & Wilkins, Philadelphia, pp 39–62.
Knolle PA, Gerken G 2000 Local control of the immune response in the liver. Immunol Rev 174: 21–34.
Bezerra JA, Tiao G, Ryckman FC, Alonso M, Sabla GE, Shneider B, Sokol RJ, Aronow BJ 2002 Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet 360: 1653–1659.
Altman JD, Lippolis JD 2001 Cytotoxic T-cell function. In: Rich RR, Fleisher TA, Shearer WT, Kotzin BL, Schroeder HW Jr (eds) Clinical Immunology Principles and Practice. Mosby, London, pp 17.1–17.9
Ohya T, Fujimoto T, Shimomura H, Miyano T 1995 Degeneration of intrahepatic bile ducts with lymphocyte infiltration into biliary epithelial cells in biliary atresia. J Pediatr Surg 30: 515–518.
Muraji T, Higashimoto Y 1997 The improved outlook for biliary atresia with corticosteroid therapy. J Pediatr Surg 32: 1103–1107.
Dillon PW, Owings E, Cilley R, Field D, Curnow A, Georgeson K 2001 Immuno-suppression as adjuvant therapy for biliary atresia. J Pediatr Surg 36: 80–85.
Meyers RL, Book LS, O'Gorman MA, Jackson WD, Black RE, Johnson DG, Matlak ME 2003 High-dose steroids, ursodeoxycholic acid, and chronic intravenous antibiotics improve bile flow after Kasai procedure in infants with biliary atresia. J Pediatr Surg 38: 406–411.
Janeway CA, Travers P, Walport M, Shlomchik 2001 Immunobiology 5: The Immune System in Health and Disease. Garland Publishing, New York, pp 554–555.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the National Institutes of Health Grant KO8 DK60710-02, National Institute of Neurological Disorders and Stroke Grant R01NS-23349, Glaxo-SmithKline Institute for Digestive Health 2002 Basic Science Research Award, Johnny Jenna Foundation (Brookfield, IL, U.S.A.), and Liver Foundation for Kids (Lemont, IL, U.S.A.).
Rights and permissions
About this article
Cite this article
Mack, C., Tucker, R., Sokol, R. et al. Biliary Atresia Is Associated with CD4+ Th1 Cell–Mediated Portal Tract Inflammation. Pediatr Res 56, 79–87 (2004). https://doi.org/10.1203/01.PDR.0000130480.51066.FB
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000130480.51066.FB
This article is cited by
-
Technical optimization of spatially resolved single-cell transcriptomic datasets to study clinical liver disease
Scientific Reports (2024)
-
Development of liver inflammatory injury in biliary atresia: from basic to clinical research
Pediatric Surgery International (2023)
-
IL13 and periostin in active fibrogenic areas of the extrahepatic bile ducts in biliary atresia patients
Pediatric Surgery International (2022)
-
Programmed cell death protein-1 (PD-1) protects liver damage by suppressing IFN-γ expression in T cells in infants and neonatal mice
BMC Pediatrics (2021)
-
RRV-induced biliary atresia in neonatal mice involves CD8 + T lymphocyte killer cells and the Notch signaling pathway
Genes & Genomics (2021)