Abstract
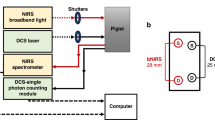
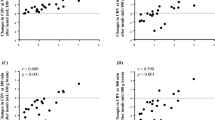
A new technique known as tissue dye densitometry (TDD) has been developed to simultaneously measure cerebral blood volume (CBV) and total circulating blood volume (TCV) using near infrared (NIR) spatially resolved spectroscopy (SRS) and the injection of indocyanine green (ICG). Using a medical NIR spectrometer with SRS capability (NIRO-300, Hamamatsu KK), a new parameter is calculated known as the ICG Hb index (IHI), which represents the ratio of ICG concentration to Hb concentration in tissue. Acting as a tracer, ICG is cleared by the liver over 15 min, providing a change of tracer concentration (ΔCICG,tis), which allows the calculation of the total Hb concentration in tissue (tcHb) using the equation:tcHbtis (μ molar) = ΔCICG,tis/ΔIHI. The CBV can subsequently be calculated from tcHbtis given the absolute Hb concentration in blood (g/dL), from which the ICG concentration in blood (ΔCICG,bl) is obtained. By back-extrapolating the ΔCICG,bl curve to the peak time, the initial ICG concentration in tissue blood (C0ICG,bl) can be found and TCV can then be calculated. The TCV of 17 neonates were measured using the TDD technique and for comparison using the previously reported fetal Hb dilution technique (FHD). The mean TCV measured by the FHD and TDD techniques were 70.19 ± 13.73 mL/kg and 70.80 ± 32.54 mL/kg. The Bland Altman plot showed that the bias was 0.61 ± 34.34 mL/kg and limits of agreement (2 SD) were −68.07 mL/kg and 69.30 mL/kg. The agreement is limited and the TDD technique needs further validation and development for use in a clinical environment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CBV:
-
cerebral blood volume
- DPF:
-
differential pathlength factor
- FHD:
-
fetal Hb dilution
- ICG:
-
indocyanine green
- IHI:
-
ICG Hb index
- NIR:
-
near infrared
- PDD:
-
pulse dye densitometry
- SRS:
-
spatially resolved spectroscopy
- TCV:
-
total circulating blood volume
- TDD:
-
tissue dye densitometry
REFERENCES
Narendra A, Aitchison TC, Beckett C, Coutts J, Holland BM, Jones JG, Kyle BM, Turner TL, Wardrop CA 1998 Can we assess the preterm infant's blood volume status clinically. Proc Royal Coll Paediatr Child Health 2: 61
Shippy CR, Appel PL, Shoemaker WC 1984 Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit Care Med 12: 107–112
Hudson IR, Cavill IA, Cooke A, Holland BM, Hoy TG, Trevett D, Turner TL, Wardrop CA 1990 Biotin labelling of red cells in the measurement of red cell volume in preterm infants. Pediatr Res 28: 199–202
Anthony MY, Goodall SR, Papouli M, Levene MI 1992 Measurement of plasma volume in neonates. Arch Dis Child 67: 36–40
Matcher SJ, Kirkpatrick P, Nahid K, Cope M, Delpy DT 1995 Absolute quantification methods in tissue near infrared spectroscopy. Proc SPIE 2389: 486–495
Suzuki S, Takasaki S, Ozaki T, Kobayashi Y 1999 A Tissue Oxygenation Monitor using NIR Spatially Resolved Spectroscopy. Proc SPIE 3597: 582–592
Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO 1988 Cotside measurement of cerebral blood flow in ill newborn infants by near-infrared spectroscopy. Lancet 2: 770–771
Patel J, Marks K, Roberts I, Azzopardi D, Edwards AD 1998 Measurement of cerebral blood flow in newborn infants using near infrared spectroscopy with indocyanine green. Pediatr Res 43: 34–39
Wyatt JS, Cope M, Delpy DT, Richardson CE, Edwards AD, Wray S, Reynolds OR 1990 Quantitation of cerebral blood volume in human infants by near-infrared spectroscopy. J Appl Physiol 68: 1086–1091
Hopton P, Walsh TS, Lee A 1999 Measurement of cerebral blood volume using near-infrared spectroscopy and indocyanine green elimination. J Appl Physiol 87: 1981–1987
Cope M, Delpy DT 1988 A system for the long term measurement of cerebral blood and tissue oxygenation in newborn infants by near infrared transillumination. Med Biol Eng Comput 26: 289–294
Phillips HM, Holland BM, Abdel-Moiz A, Fayed S, Jones JG, Turner TL, Wardrop CA, Cockburn F 1986 Determination of red-cell mass in assessment and management of anaemia in babies needing blood transfusion. Lancet 8464: 882–884
De Visscher G, van Rossem K, van Reempts JV, Borgers M, Flameng W, Reneman RS 2002 Cerebral blood flow assessment with indocyanine green bolus transit detection by near-infrared spectroscopy in the rat. Biochem Physiol 132: 87–95
Delpy DT, Cope M 1997 Quantification in tissue near-infrared spectroscopy. Philos Trans R Soc Lond B Biol Sci 352: 649–659
Duncan A, Meek JH, Clemence M, Elwell CE, Fallon P, Tyszczuk L, Cope M, Delpy DT 1996 Measurement of cranial optical path length as a function of age using phase resolved near infrared spectroscopy. Pediatr Res 39: 889–894
Matcher SJ, Cope M, Delpy DT 1993 Use of the water absorption spectrum to quantify tissue chromophore concentration changes in near-infrared spectroscopy. Phys Med Biol 38: 177–196
van der Zee P 1993 Measurement and modelling of the optical properties of biological tissues in the near infrared. PhD thesis, University of London
Woodward HQ, White DR 1986 The composition of body tissues. Br J Radiol 59: 1209–1219
Cooper CE, Elwell CE, Meek JH, Matcher SJ, Wyatt JS, Cope M, Delpy DT 1996 The non-invasive measurement of absolute cerebral deoxyhemoglobin concentration and mean optical path length in the neonatal brain by second derivative near infrared spectroscopy. Pediatr Res 39: 32–38
Lammertsma AA, Brooks DJ, Beaney RP, Turton DR, Kensett MJ, Heather JD, Marshall J, Jones T 1984 In vivo measurement of regional cerebral haematocrit using positron emission tomography. J Cereb Blood Flow Metab 4: 317–322
Iijima T, Aoyagi T, Iwao Y, Junichi M, Fuse M, Kobayashi N, Sankawa H 1997 Cardiac output and circulating blood volume analysis by pulse dye-densitometry. J Clin Monit 13: 81–89
Picker O, Wietasch G, Scheeren TW, Arndt JO 2001 Determination of total blood volume by indicator dilution: a comparison of mean transit time and mass conservation principle. Intensive Care Med 27: 767–774
Kutlar A, Kutlar F, Wilson JB, Headlee MG, Huisman TH 1984 Quantitation of hemoglobin components by high-performance cation-exchange liquid chromatography: its use in diagnosis and in the assessment of cellular distribution of hemoglobin variants. Am J Hematol 17: 39–53
Bland JM, Altman DG 1986 Statistical methods for assessing agreement between two methods of clinical measurements. Lancet 8: 307–310
Caesar J, Shaldon S, Chiandussi L, Guevara L, Sherlock S 1961 The use of indocyanine green in the measurement of hepatic blood flow and as a test of hepatic function. Clin Sci 21: 43–57
Emmanouilides GC, Moss AJ, Monset-Couchard M, Marcano BA, Rzeznic B 1970 Cardiac output in newborn infants. Biol Neonat 15: 186–197
Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA 1993 Adverse reactions due to indocyanine green. Ophthalmology 101: 529–533
Sakai F, Nakazawa K, Tazaki Y, Ishii K, Hino H, Igarashi H, Kanda T 1985 Regional cerebral blood volume and haematocrit measured in normal human volunteers by single-photon emission computed tomography. J Cereb Blood Flow Metab 5: 207–213
Mollison PL, Veall N, Cutbush M 1950 Red cell and plasma volume in newborn infants. Arch Dis Child 25: 242–253
Leipala JA, Talme M, Viitala J 1999 Determination of neonatal blood volume with the haemoglobin subtype method in appropriately grown and growth retarded preterm infants. Pediatr Res 45: 206A
Ladurner G, Zilkha E, Iliff D, Du Boulay GH, Marshall J 1976 Measurement of regional cerebral blood volume by computerised axial tomography. J Neurol Neurosurg Psychiatry 39: 152–158
Okada E, Delpy DT 1996 The effect of overlying tissue on NIR light propagation in neonatal brain. OSA TOPS on Advances in Optical Imaging Photon Migration 2: 338–343
Imai T, Mitaka C, Koike A, Ohki S, Isa Y, Kunimoto F 2000 Accuracy and repeatability of blood volume measurement by pulse dye densitometry compared to the conventional method using 51Cr-labeled red blood cells. Intensive Care Med 26: 1343–1349
Matcher SJ, Elwell CE, Cooper CE, Cope M, Delpy DT 1995 Performance comparison of several published tissue near-infrared spectroscopy algorithms. Anal Biochem 227: 54–68
Acknowledgements
The authors would like to thank all the patients and their parents participated in this study, medical and nursing staff in the NICU and the department of hematology Homerton University Hospital for their cooperation, the Wellcome Trust for funding (TSL), and Hamamatsu KK for providing a spectrometer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Funded by a grant from the Wellcome Trust (Grant 054762).
APPENDIX
APPENDIX
It has been reported that the preterm neonatal brain consists of 90% water (19) and the background optical absorption in cerebral tissues account for approximately 30% of the total absorption coefficient (16, 17). This background absorption includes all sorts of chromophores found in tissue including melanin, lipids and etc. The background absorption appears as a constant offset in the absorption coefficient over all wavelengths. In general in the NIR spectroscopy, we can consider 5 major chromophores in tissue: oxy-Hb, deoxy-Hb, ICG, water and background substances. Based on the modified Beer-Lambert law, we can write:

where μ^a(λ1) is the absorption coefficient at wavelength λ1, ε is the specific extinction coefficient of a chromophore, C is the concentration of the chromophore and b is a constant absorption coefficient representing the background absorption. In matrix form,

where

Using published data and data measured in our laboratory for the specific extinction coefficients for Hb (34, ICG and water (λ1=778 nm, λ2=813 nm, λ3=850 nm):

In this paper, we consider the background absorption to account for 22% of μ^a at all wavelengths and can be approximated by:

The NIRO-300 spectrometer used in these studies includes a measurement at a wavelength at 813 nm. The effect of the background absorption can be minimized by subtracting it from the calculated total μa. Equation 1 is now rewritten as:

where represents the absorption estimated to arise from the four known chromophores. The NIRO-300 spectrometer uses a measurement of attenuation gradients at 3 wavelengths to calculate μ^. The concentrations of the 3 chromophores (HHb, HbO2, and ICG) can then be estimated using least square minimization.

where Ĉ is the estimated concentrations and εR−1 is the inverse of the reduced matrix. Both matrices account for only HHb, HbO2, and ICG:

The estimated concentration based on a 3-wavelength SRS spectrometer becomes:

Solving the simultaneous equations results in,

It can be seen from Equation 7 that the estimated concentrations are affected by the volume assumed for the water content of the tissue. Assuming 90% water in neonatal brains (recall 100% pure water corresponds to 55.4 Molar), CH2O can be calculated

Substituting Equations 7 and 8 into the expression for ΔIHI, we obtain:

The above result shows that if no correction is made for the effects of water absorption, then using ΔIHI and ΔCICG to estimate tcHb will lead to an over-estimate of 15.15 μ molar. To compensate for this offset, in this paper the value obtained from the expression tcHb = ΔCICG/ΔIHI has been corrected by subtracting 15.15 μ mol. To assess the sensitivity of the results obtained to the assumptions made in these calculations, we have looked at the sensitivity of the correlation coefficient and the mean difference between TCVTDD and TCVFHD over a range of different values of water content (70% to 95%) and assumed background absorption contribution (5% to 35%). A high correlation coefficient (>0.8) was maintained with water content variation over the whole range (70% to 95%) and for background absorptions of <35%. The mean difference was as high as 42.09 mL with the rather un-physiologic assumptions of a background absorption = 5% and water content = 70%. The mean difference changed by −1.68 mL per % change in background absorption and by −0.70 mL per % for the water content. The minimum mean difference (0.0219 mL) was obtained when the water content = 85% and the background absorption = 24%, close to the values of 90% and 22% used in this paper and derived from independently measured data (16, 19).
Rights and permissions
About this article
Cite this article
Leung, T., Aladangady, N., Elwell, C. et al. A New Method for the Measurement of Cerebral Blood Volume and Total Circulating Blood Volume Using Near Infrared Spatially Resolved Spectroscopy and Indocyanine Green: Application and Validation in Neonates. Pediatr Res 55, 134–141 (2004). https://doi.org/10.1203/01.PDR.0000099775.87684.FB
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000099775.87684.FB
This article is cited by
-
Relationship of cerebral blood volume with arterial and venous flow velocities in extremely low-birth-weight infants
European Journal of Pediatrics (2023)
-
Preservation of the metabolic rate of oxygen in preterm infants during indomethacin therapy for closure of the ductus arteriosus
Pediatric Research (2013)