Abstract
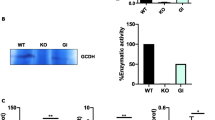
To assess the role of brain antioxidant capacity in the pathogenesis of neonatal hypoxic-ischemic brain injury, we measured the activity of glutathione peroxidase (GPX) in both human-superoxide dismutase-1 (hSOD1) and human-GPX1 overexpressing transgenic (Tg) mice after neonatal hypoxia-ischemia (HI). We have previously shown that mice that overexpress the hSOD1 gene are more injured than their wild-type (WT) littermates after HI, and that H2O2 accumulates in HI hSOD1-Tg hippocampus. We hypothesized that lower GPX activity is responsible for the accumulation of H2O2. Therefore, increasing the activity of this enzyme through gene manipulation should be protective. We show that brains of hGPX1-Tg mice, in contrast to those of hSOD-Tg, have less injury after HI than WT littermates: hGPX1-Tg, median injury score = 8 (range, 0–24) versus WT, median injury score = 17 (range, 2–24), p < 0.01. GPX activity in hSOD1-Tg mice, 2 h and 24 h after HI, showed a delayed and bilateral decline in the cortex 24 h after HI (36.0 ± 1.2 U/mg in naive hSOD1-Tg versus 29.1 ± 1.7 U/mg in HI cortex and 29.2 ± 2.0 for hypoxic cortex, p < 0.006). On the other hand, GPX activity in hGPX1-Tg after HI showed a significant increase by 24 h in the cortex ipsilateral to the injury (48.5 ± 5.2 U/mg, compared with 37.2 ± 1.5 U/mg in naive hGPX1-Tg cortex, p < 0.008). These findings support the hypothesis that the immature brain has limited GPX activity and is more susceptible to oxidative damage and may explain the paradoxical effect seen in ischemic neonatal brain when SOD1 is overexpressed.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CCA:
-
common carotid artery
- GPX:
-
glutathione peroxidase
- GSH:
-
glutathione
- HI:
-
hypoxia-ischemia
- P:
-
postnatal day
- red-ox:
-
oxidation-reduction reaction
- ROS:
-
reactive oxygen species
- SOD1:
-
copper-zinc superoxide dismutase
- Tg:
-
transgenic
- WT:
-
wild-type
References
Aspberg A, Tottmar O 1992 Development of antioxidant enzymes in rat brain and in reaggregation culture of fetal brain cells. Brain Res Dev Brain Res 66: 55–58
Mavelli I, Rigo A, Federico R, Ciriolo M, Rotilio G 1982 Superoxide dismutase, glutathione peroxidase and catalase in developing rat brain. Biochem J 204: 535–540
Buard A, Clement M, Bourre J 1992 Developmental changes in enzymatic systems involved in protection against peroxidation in isolated rat brain microvessels. Neurosci Lett 141: 72–74
Khan JY, Black SM 2003 Developmental changes in murine brain antioxidant enzymes. Pediatr Res 54: 77–82
de Haan JB, Bladier C, Griffiths P, Kelner M, O'Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS, Beart PM, Hertzog PJ, Kola I 1998 Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem 273: 22528–22536
Fan P, Yamauchi T, Noble LJ, Ferriero DM 2003 Age-dependent differences in glutathione peroxidase activity after traumatic brain injury. J Neurotrauma 20: 437–445
Guegan C, Ceballos-Picot I, Nicole A, Kato H, Onteniente B, Sola B 1998 Recruitment of several neuroprotective pathways after permanent focal ischemia in mice. Exp Neurol 154: 371–380
Goss JR, Taffe KM, Kochanek PM, DeKosky ST 1997 The antioxidant enzymes glutathione peroxidase and catalase increase following traumatic brain injury in the rat. Exp Neurol 146: 291–294
Yang G, Chan PH, Chen J, Carlson E, Chen SF, Weinstein P, Epstein CJ, Kamii H 1994 Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25: 165–170
Ditelberg J, Sheldon RA, Epstein CJ, Ferriero DM 1996 Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res 39: 204–208
Mischel RE, Kim YS, Sheldon RA, Ferriero DM 1997 Hydrogen peroxide is selectively toxic to immature murine neurons in vitro. Neurosci Lett 231: 17–20
Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, Ferriero DM 1998 Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol 44: 357–364
Almli LM, Hamrick SE, Koshy AA, Täuber MG, Ferriero DM 2001 Multiple pathways of neuroprotection against oxidative stress and excitotoxic injury in immature primary hippocampal neurons. Brain Res Dev Brain Res 132: 121–129
Ishibashi N, Prokopenko O, Weisbrot-Lefkowitz M, Reuhl KR, Mirochnitchenko O 2002 Glutathione peroxidase inhibits cell death and glial activation following experimental stroke. Brain Res Mol Brain Res 109: 34–44
Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O 1998 Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res 53: 333–338
Sheldon RA, Almli L, Ferriero DM 2002 Copper/zinc superoxide dismutase transgenic brain in neonatal hypoxia-ischemia. Methods Enzymol 353: 389–397
Epstein CJ, Avraham KB, Lovett M, Smith S, Elroy-Stein O, Rotman G, Bry C, Groner Y 1987 Transgenic mice with increased Cu/Zn-superoxide dismutase activity: animal model of dosage effects in Down syndrome. Proc Natl Acad Sci U S A 84: 8044–8048
Chan PH, Epstein CJ, Li Y, Huang TT, Carlson E, Kinouchi H, Yang G, Kamii H, Mikawa S, Kondo T, et al. 1995 Transgenic mice and knockout mutants in the study of oxidative stress in brain injury. J Neurotrauma 12: 815–824
Huang TT, Raineri I, Eggerding F, Epstein CJ 2002 Transgenic and mutant mice for oxygen free radical studies. Methods Enzymol 349: 191–213
Mirault ME, Tremblay A, Furling D, Trepanier G, Dugre F, Puymirat J, Pothier F 1994 Transgenic glutathione peroxidase mouse models for neuroprotection studies. Ann N Y Acad Sci 738: 104–115
Rice JE III, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol 9: 131–141
Sarco DP, Becker J, Palmer C, Sheldon RA, Ferriero DM 2000 The neuroprotective effect of deferoxamine in the hypoxic-ischemic immature mouse brain. Neurosci Lett 282: 113–116
Roveri A, Maiorino M, Ursini F 1994 Enzymatic and immunological measurements of soluble and membrane-bound phospholipid-hydroperoxide glutathione peroxidase. Methods Enzymol 233: 202–212
Flohé L, Günzler WA 1984 Assays of glutathione peroxidase. Methods Enzymol 105: 114–121
Ishibashi N, Prokopenko O, Reuhl K, Mirochnitchenko O 2002 Inflammatory response and glutathione peroxidase in a model of stroke. J Immunol 168: 1926–1933
Keller JN, Huang FF, Zhu H, Yu J, Ho YS, Kindy TS 2000 Oxidative stress-associated impairment of proteasome activity during ischemia-reperfusion injury. J Cereb Blood Flow Metab 20: 1467–1473
Furling D, Ghribi O, Lahsaini A, Mirault ME, Massicotte G 2000 Impairment of synaptic transmission by transient hypoxia in hippocampal slices: improved recovery in glutathione peroxidase transgenic mice. Proc Natl Acad Sci U S A 97: 4351–4356
Klivenyi P, Andreassen O, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF 2000 Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci 20: 1–7
Crack PJ, Taylor JM, Flentjar NJ, de Haan J, Hertzog P, Iannello RC, Kola I 2001 Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J Neurochem 78: 1389–1399
Crack PJ, Taylor JM, de Haan JB, Kola I, Hertzog P, Iannello RC 2003 Glutathione peroxidase-1 contributes to the neuroprotection seen in the superoxide dismutase-1 transgenic mouse in response to ischemia/reperfusion injury. J Cereb Blood Flow Metab 23: 19–22
Martensson J, Meister A, Mrtensson J 1991 Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A 88: 6898
Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H 1998 Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 17: 2596–2606
Wilhelm D, Bender K, Knebel A, Angel P 1997 The level of intracellular glutathione is a key regulator for the induction of stress-activated signal transduction pathways including Jun N-terminal protein kinases and p38 kinase by alkylating agents. Mol Cell Biol 17: 4792–4800
Kretz-Remy C, Mehlen P, Mirault M, Arrigo AP 1996 Inhibition of I kappa B-alpha phosphorylation and degradation and subsequent NF-kappa B activation by glutathione peroxidase overexpression. J Cell Biol 133: 1083–1093
Li X, Song L, Jope RS 1998 Glutathione depletion exacerbates impairment by oxidative stress of phosphoinositide hydrolysis, AP-1, and NF-kappa B activation by cholinergic stimulation. Brain Res Mol Brain Res 53: 196–205
Cho S, Hazama M, Urata Y, Goto S, Horiuchi S, Sumikawa K, Kondo T 1999 Protective role of glutathione synthesis in response to oxidized low density lipoprotein in human vascular endothelial cells. Free Radic Biol Med 26: 589–602
Drukarch B, Schepens E, Jongenelen C, Stoof JC, Langeveld CH 1997 Astrocyte-mediated enhancement of neuronal survival is abolished by glutathione deficiency. Brain Res 770: 123–130
Wüllner U, Seyfried J, Groscurth P, Beinroth S, Winter S, Gleichmann M, Heneka M, Loschmann P, Schulz JB, Weller M, Klockgether T 1999 Glutathione depletion and neuronal cell death: the role of reactive oxygen intermediates and mitochondrial function. Brain Res 826: 53–62
Sies H, Klotz LO, Sharov VS, Assmann A, Briviba K 1998 Protection against peroxynitrite by selenoproteins. Z Naturforsch 53: 228–232
Francescutti D, Baldwin J, Lee L, Mutus B 1996 Peroxynitrite modification of glutathione reductase: modeling studies and kinetic evidence suggest the modification of tyrosines at the glutathione disulfide binding site. Protein Eng 9: 189–194
Francis JW, Ren J, Warren L, Brown RH Jr, Finklestein SP 1997 Postischemic infusion of Cu/Zn superoxide dismutase or SOD: Tet451 reduces cerebral infarction following focal ischemia/reperfusion in rats. Exp Neurol 146: 435–443
Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko T, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP 1999 A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science 286: 304–306
Shimizu K, Rajapakse N, Horiguchi T, Payne RM, Busija DW 2003 Neuroprotection against hypoxia-ischemia in neonatal rat brain by novel superoxide dismutase mimetics. Neurosci Lett 346: 41–44
Mollace V, Iannone M, Muscoli C, Palma E, Granato T, Modesti A, Nistico R, Rotiroti D, Salvemini D 2003 The protective effect of M40401, a superoxide dismutase mimetic, on post-ischemic brain damage in Mongolian gerbils. BMC Pharmacol 3: 8
Dawson DA, Masayasu H, Graham DI, Macrae IM 1995 The neuroprotective efficacy of ebselen (a glutathione peroxidase mimic) on brain damage induced by transient focal cerebral ischaemia in the rat. Neurosci Lett 185: 65–69
Imai H, Graham DI, Masayasu H, Macrae IM 2003 Antioxidant ebselen reduces oxidative damage in focal cerebral ischemia. Free Radic Biol Med 34: 56–63
Guerin PJ, Gauthier ER 2003 Induction of cellular necrosis by the glutathione peroxidase mimetic ebselen. J Cell Biochem 89: 203–211
Peeters-Scholte C, Braun K, Koster J, Kops N, Blomgren K, Buonocore G, Van Buul-Offers S, Hagberg H, Nicolay K, van Bel F, Groenendaal F 2003 Effects of allopurinol and deferoxamine on reperfusion injury of the brain in newborn piglets after neonatal hypoxia-ischemia. Pediatr Res 54: 516–522
Palmer C, Vannucci RC, Towfighi J 1990 Reduction of perinatal hypoxic-ischemic brain damage with allopurinol. Pediatr Res 27: 332–336
Palmer C, Towfighi J, Roberts RL, Heitjan DF 1993 Allopurinol administered after inducing hypoxia-ischemia reduces brain injury in 7-day-old rats. Pediatr Res 33: 405–411
Palmer C, Roberts RL, Bero C 1994 Deferoxamine posttreatment reduces ischemic brain injury in neonatal rats. Stroke 25: 1039–1045
Whitelaw A, Thoresen M 2002 Clinical trials of treatments after perinatal asphyxia. Curr Opin Pediatr 14: 664–668
Hamrick SE, Ferriero DM 2003 The injury response in the term newborn brain: can we neuroprotect?. Curr Opin Neurol 16: 147–154
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Institutes of Health grant NS 33997.
Rights and permissions
About this article
Cite this article
Sheldon, R., Jiang, X., Francisco, C. et al. Manipulation of Antioxidant Pathways in Neonatal Murine Brain. Pediatr Res 56, 656–662 (2004). https://doi.org/10.1203/01.PDR.0000139413.27864.50
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000139413.27864.50
This article is cited by
-
Regionally Impaired Redox Homeostasis in the Brain of Rats Subjected to Global Perinatal Asphyxia: Sustained Effect up to 14 Postnatal Days
Neurotoxicity Research (2018)
-
Paraquat and Maneb Exposure Alters Rat Neural Stem Cell Proliferation by Inducing Oxidative Stress: New Insights on Pesticide-Induced Neurodevelopmental Toxicity
Neurotoxicity Research (2018)
-
Effect of Resveratrol on Oxidative Stress and Mitochondrial Dysfunction in Immature Brain during Epileptogenesis
Molecular Neurobiology (2018)
-
Acute maternal oxidant exposure causes susceptibility of the fetal brain to inflammation and oxidative stress
Journal of Neuroinflammation (2017)
-
Glucose and Intermediary Metabolism and Astrocyte–Neuron Interactions Following Neonatal Hypoxia–Ischemia in Rat
Neurochemical Research (2017)