Abstract
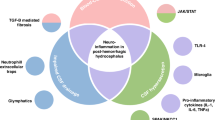
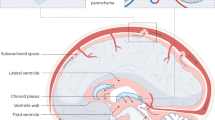
The expression of specific growth factors such as vascular endothelial growth factor (VEGF) and transforming growth factor-β1 (TGF-β1) is of importance during brain development and in the pathogenesis of neurodegenerative disorders. VEGF and TGF-β1 was studied in the cerebrospinal fluid (CSF) of neonates with posthemorrhagic hydrocephalus (PHHC) and nonhemorrhagic hydrocephalus. For determining the interference of inflammatory cytokine interaction with the expression of VEGF and TGF-β1, IL-6 and IL-10 CSF concentrations were measured. Eighteen neonates who had PHHC and underwent serial reservoir puncture and nine neonates who had congenital nonhemorrhagic hydrocephalus (CHC) and underwent first shunt surgery were included in the study. CSF samples of 11 neonates with lumbar puncture for the exclusion of meningitis served as control subjects. VEGF, TGF-β1, IL-6, and IL-10 concentrations in the CSF were measured by ELISA technique. VEGF concentrations in the CSF of patients with PHHC were significantly higher (median: 377 pg/mL; range: 101–1301 pg/mL) when compared with patients with CHC (median: 66 pg/mL; range: 3–1991; p < 0.001) and control subjects (median: 2 pg/mL; range: 0–12 pg/mL; p < 0.0001). TGF-β1 CSF concentrations did not differ from control infants in all groups. Median IL-6 and IL-10 concentrations in the CSF were found to be low in all patient groups. Increased release of VEGF in the CSF of neonates with PHHC and nonhemorrhagic hydrocephalus may serve as an indicator of brain injury from progressive ventricular dilation. TGF-β1 CSF concentrations are not elevated in the phase of acute fibroproliferative reactions in patients with PHHC.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- CHC:
-
congenital hydrocephalus
- CSF:
-
cerebrospinal fluid
- ICH:
-
intracerebral hemorrhage
- ICP:
-
intracerebral pressure
- IVH:
-
intraventricular hemorrhage
- PHHC:
-
posthemorrhagic hydrocephalus
- TGF-β1:
-
transforming growth factor-β1
- VEGF:
-
vascular endothelial growth factor
- WMD:
-
white matter disease
References
Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefiled LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS 1986 Transforming growth factor type β rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83: 4167–4171
Ignotz RA, Massague J 1986 Transforming growth factor β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261: 4337–4345
Arai Y, Deguchi K, Takashima S 1998 Vascular endothelial growth factor in brains with periventricular leukomalacia. Pediatr Neurol 19: 45–49
Cheng SY, Nagane M, Huang HS, Cavenee WK 1997 Intracerebral tumor associated hemorrhage caused by overexpression of vascular endothelial growth factor isoforms VEGF 121 and VEGF 165 but not VEGF 189. Proc Natl Acad Sci USA 94: 12081–12087
Jones KL, Krous HF, Nadeau J, Blackbourne B, Zielke HR, Gozal D 2003 Vascular endothelial growth factor in the cerebrospinal fluid of infants who died of sudden infant death syndrome: evidence for antecedent hypoxia. Pediatrics 111: 358–363
Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LE 1995 Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci USA 31: 905–909
Blobe GC, Schiemann WP, Lodish HF 2000 Role of transforming growth factor beta in human disease. N Engl J Med 342: 1350–1358
Johnson MD, Gold LI, Moses HL 1992 Evidence for transforming growth factor β expression in human leptomeningeal cells and transforming growth factor β-like activity in human cerebrospinal fluid. Lab Invest 67: 360–368
Massicotte EM, Del Bigio MR 1999 Human arachnoid villi response to subarachnoideal hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg 91: 80–84
Kim RC, Talbert WM, Choe W, Choi BH 1989 Massive craniospinal collagen deposition after persistent postoperative intraventricular bleeding. Neurosurgery 24: 771–775
Volpe JJ 2001 Intracranial hemorrhage: germinal matrix-intraventricular hemorrhage of the premature infant. In: Neurology of the Newborn. WB Saunders, Philadelphia, pp 428–493
Heep A, Stoffel-Wagner B, Soditt V, Aring C, Groneck P, Bartmann P 2002 Procollagen I C-propeptide in the cerebrospinal fluid of neonates with posthemorrhagic hydrocephalus. Arch Dis Child Fetal Neonatal Ed 87: F34–F36
Heep A, Stoffel-Wagner B, Bartmann P 2000 Procollagen propeptides in the cerebrospinal fluid of neonates with posthemorrhagic hydrocephalus (PHHC). Biol Neonate 78: 153
Paneth N 1999 Classifying brain damage in preterm infants. J Pediatr 134: 527–529
Levene MI 1981 Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child 56: 900–904
Heep A, Engelskirchen R, Holschneider A, Groneck P 2001 Primary intervention for posthemorrhagic hydrocephalus in very low birthweight infants by ventriculostomy. Childs Nerv Syst 17: 47–51
Segura I, Serrano A, Buitrago GG, Gonzales MA, Abad JL, Claveria C, Gomez L, Bernad A, Martinez AC, Riese HH 2002 Inhibition of programmed cell death impairs in vitro vascular-like structure formation and reduces in vivo angiogenesis. FASEB J 16: 833–841
Flood C, Akinwunmi J, Lagord C, Daniel M, Berry M, Jackowski A, Logan A 2001 Transforming growth factor-beta1 in the cerebrospinal fluid of patients with subarachnoid hemorrhage: titers derived from exogenous and endogenous sources. J Cereb Blood Flow Metab 21: 157–162
Kitizawa K, Tada T 1994 Elevation of transforming growth factor β-1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke 25: 1400–1404
Scheufler KM, Drevs J, van Velthoven V, Reusch P, Klisch J, Augustin HG, Zentner J, Marme D 2003 Implications of vascular endothelial growth factor, sFlt-1, and sTie-2 in plasma, serum and cerebrospinal fluid during cerebral ischemia in man. J Cereb Blood Flow Metab 23: 99–110
Slevin M, Krupinski J, Slowik A, Kumar P, Szczudlik A, Gaffney J 2000 Serial measurement of vascular endothelial growth factor and transforming growth factor-beta 1 in serum of patients with acute ischemic stroke. Stroke 31: 1863–1870
Ueberham U, Ueberham E, Gruschka H, Arendt T 2003 Connective tissue growth factor in Alzheimer's disease. Neuroscience 116: 1–6
Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P 2002 Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol Aging 23: 237–243
Bocker-Meffert S, Rosenstiel P, Rohl C, Warneke N, Held-Feindt J, Sievers J, Lucius R 2002 Erythropoietin and VEGF promote neural outgrowth from retinal explants in postnatal rats. Invest Ophthalmol Vis Sci 43: 2021–2026
Koehne P, Hochhaus F, Felderhoff-Mueser U, Ring-Mrozik E, Obladen M, Buhrer C 2002 Vascular endothelial growth factor and erythropoietin concentrations in cerebrospinal fluid of children with hydrocephalus. Childs Nerv Syst 18: 137–141
Leviton A, Dammann O, O'Shea TM, Paneth N 2002 Adult stroke and perinatal brain damage: like grandparent, like grandchild?. Neuropediatrics 33: 281–287
Nakazato F, Tada T, Sekiguchi Y, Murakami K, Yanagisawa S, Tanaka Y, Hongo K 2002 Disturbed spatial learning of rats after intraventricular administration of transforming growth factor-beta 1. Neurol Med Chir (Tokyo) 42: 151–156
Moinuddin SM, Tada T 2000 Study of cerebrospinal fluid flow dynamics in TGFβ1 induced chronic hydrocephalic mice. Neurol Res 22: 215–222
Ilzecka J, Stelmasiak Z, Dobosz B 2002 Transforming growth factor-beta 1 (TGF-beta 1) in patients with amyotrophic lateral sclerosis. Cytokine 20: 239–243
Rollnik JD, Sindern E, Schweppe C, Malin JP 1997 Biologically active TGF-beta 1 is increased in cerebrospinal fluid while it is reduced in serum in multiple sclerosis patients. Acta Neurol Scand 96: 101–105
Zhu Y, Yang GY, Ahlemeyer B, Pang L, Che XM, Culmsee C, Klumpp S, Krieglstein J 2002 Transforming growth factor–beta 1 increases bad phosphorylation and protects neurons against damage. J Neurosci 15: 3898–3909
Buisson A, Lesne S, Docagne F, Ali C, Nicole O, MacKenzie ET, Vivien D 2003 Transforming growth factor–beta and ischemic brain injury. Cell Mol Neurobiol 23: 539–550
Whitelaw A, Christie S, Pople I 1999 Transforming growth factor–beta 1: a possible signal molecule for posthemorrhagic hydrocephalus?. Pediatr Res 46: 576–580
Csuka E, Morganti-Kossmann MC, Lenzlinger PM, Joller H, Trentz O, Kossmann T 1999 IL-10 levels in cerebrospinal fluid and serum of patients with severe traumatic brain injury: relationship to IL-6, TNF-alpha, TGF-beta1 and blood-brain barrier function. J Neuroimmunol 101: 211–221
Schromm AB, Brandenburg K, Loppnow H, Zähringer U, Rietschel ET, Carroll SF, Koch MH, Kusumoto S, Seydel U 1998 The charge of endotoxin molecules influences their conformation and IL-6-inducing capacity. J Immunol 161: 5464–5471
Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG 2000 Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res 47: 64–72
Cai Z, Pang Y, Lin S, Rhodes PG 2003 Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res 13: 37–47
Hagberg H, Gilland E, Bona E, Hanson LA, Hanin-Zoric M, Blennow M, Holst M, McRae A, Söder O 1996 Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia-ischemia in neonatal rats. Pediatr Res 40: 603–609
Stanimirovic D, Satoh K 2000 Inflammatory mediators of cerebral endothelium: a role of ischemic brain inflammation. Brain Pathol 10: 113–126
Sävman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H 1998 Cytokine response in the cerebrospinal fluid after birth asphyxia. Pediatr Res 43: 746–751
Heep A, Schaller C, Rittmann N, Himbert U, Marklein G, Bartmann P 2004 Multiple brain abscesses in an extremely preterm infant: treatment surveillance with interleukin-6 in the CSF. Eur J Pediatr 163: 44–45
Baumeister FA, Pohl-Koppe A, Hofer M, Kim JO, Weiss M 2000 IL-6 in CSF during ventriculitis in preterm infants with posthemorrhagic hydrocephalus. Infection 28: 234–236
Xiao L, Li XQ, Zhang HX 2002 Effects of nao-yi-an granule on the intercellular expression of IL-6 in the experimental intracerebral hemorrhagic brain of rats. Hunan Yi Ke Da Xue Xue Bao 28: 123–126
Takizawa T, Tada T, Kitazawa K, Tanaka Y, Hongo K, Kameko M, Uemura KI 2001 Inflammatory cytokine cascade released by leukocytes in cerebrospinal fluid after subarachnoid hemorrhage. Neurol Res 23: 724–730
Bell MJ, Kochanek PM, Doughty LA, Carcillo JA, Adelson PD, Clark RS, Whalen MJ, DeKosky ST 1997 Comparison of the interleukin-6 and interleukin-10 response in children after severe traumatic brain injury or septic shock. Acta Neurochir Suppl (Wien) 70: 96–97
Maskin B, Gammella D, Solari L, Videta W, Barboza MF, Geliz L, Rondina C 2001 Early release of the antiinflammatory cytokine IL-10 in traumatic brain injury. Medicina (B Aires) 61: 573–576
Acknowledgements
We gratefully acknowledge the skilled technical assistance by Anne Thiel and Martina Schmidt and the statistical support by R. Fimmers.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by BMBF grant Z 01ZZ0101.
Rights and permissions
About this article
Cite this article
Heep, A., Stoffel-Wagner, B., Bartmann, P. et al. Vascular Endothelial Growth Factor and Transforming Growth Factor-β1 Are Highly Expressed in the Cerebrospinal Fluid of Premature Infants with Posthemorrhagic Hydrocephalus. Pediatr Res 56, 768–774 (2004). https://doi.org/10.1203/01.PDR.0000141524.32142.53
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000141524.32142.53
This article is cited by
-
Pathophysiologic mechanisms and strategies for the treatment of post-hemorrhagic hydrocephalus of prematurity
Child's Nervous System (2022)
-
Biochemical profile of human infant cerebrospinal fluid in intraventricular hemorrhage and post-hemorrhagic hydrocephalus of prematurity
Fluids and Barriers of the CNS (2021)
-
Opportunities in posthemorrhagic hydrocephalus research: outcomes of the Hydrocephalus Association Posthemorrhagic Hydrocephalus Workshop
Fluids and Barriers of the CNS (2018)
-
Chemokine and cytokine levels in the lumbar cerebrospinal fluid of preterm infants with post-hemorrhagic hydrocephalus
Fluids and Barriers of the CNS (2017)
-
Nonsurgical therapy for hydrocephalus: a comprehensive and critical review
Fluids and Barriers of the CNS (2015)