Abstract
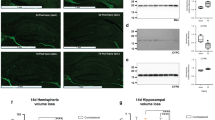
Newborn piglets were submitted to normobaric hypoxia (5% O2, 95% N2) for either 1 or 4 h. The effects of hypoxia on the neonatal brain were characterized through a time-course analysis of levels of various proteins such as heat shock proteins (HSP27, 70, and 90), hypoxia inducible factor-1α (HIF-1α), neuronal nitric oxide synthase (nNOS), hemeoxygenase-2 (HO-2), and caspase-3. The expression of these proteins was determined at different stages of recovery up to 72 h in cerebellum, cortex, and hippocampus by Western blot analysis in hypoxic maintained animals that were made hypoxic at either 20 or 37°C. In all regions of the brain, HIF-1α and HSP27 expression were strongly increased until 22 h of recovery. No significant changes were observed for HSP70, HSP90, and HO-2. A small elevation of expression of nNOS was observed at early stages in the cerebellum and the cortex with no change in the hippocampus. Expression of caspase 3 was strongly increased in the cortex 24 and 48 h after hypoxia but unchanged in the hippocampus. These results are presented in terms of the porcine model of nonischemic hypoxia and its delayed neuronal effects on the cerebral outcome. Because of their recently established biochemical and functional interactions, the expression of the main HSPs, HIF-1α, nNOS, and caspase-3 after hypoxia are delineated.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- HIF-1α:
-
hypoxia inducible factor-1α
- HO:
-
hemooxygenase
- HSP:
-
heat shock protein
- nNOS:
-
neuronal nitric oxide synthase
References
Nagdyman M, Komen W, Ko HK, Müller C, Obladen M 2001 Early biochemical indicators of hypoxia-ischemia encephalopathy after birth asphyxia. Pediatr Res 49: 502–506
David JC, Grongnet JF 2000 Effect of hypoxia on DNA fragmentation in different brain regions of the newborn piglet. Mol Reprod Dev 57: 153–158
Jiang ZD 1995 Long-term effect of perinatal and postnatal asphyxia on developing human auditory brainstem responses: peripheral hearing loss. Int J Pediatr Otorhinolaryngol 33: 225–238
Jiang ZD, Brosi DM, Wilkinson AR 2001 Comparison of brainstem auditory evoked responses recorded at different presentation rates of chicks in term neonates after asphyxia. Acta Paediatr 90: 1416–1420
Gadian DG, Aicardi J, Watkins KE, Poter DA, Mishkin M, Vargha Kadem F 2000 Developmental amnesia associated with early hypoxic-ischaemic injury. Brain 123: 499–507
Dalman C, Thomas HV, Davis AS, Gentz J, Lewis G, Allebeck P 2001 Signs of asphyxia at birth and risk of schizophrenia. Population-based control study. Br J Psychiatry 170: 403–408
Dixon G, Badawi M, Kurinczuk JJ, Keogh JM, Silburn SR, Zubrick SR, Stanley FJ 2002 Early developmental outcomes after newborn encephalopathy. Pediatrics 109: 26–33
Viapiano MS, Mitridate de Novara AM, Fiszer de Plazas S, Bozzini CE 2001 Prolonged exposure to hypobaric hypoxia transiently reduces GABAA receptor number in mice cerebral cortex. Brain Res 894: 31–36
Hsu L, Rockenstein E, Mallory M, Hashimoto M, Masliah E 2001 Altered expression of glutamate transporters under hypoxic conditions in vitro. J Neurosci Res 64: 193–202
Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS 2001 Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice. Int J Dev Neurosci 19: 197–208
Arrigo AP, Landry J 1994 Expression and function of the low molecular weight heat shock proteins. In: Morimoto L and Georgopoulos C (eds) The Biology of Heat Shock Proteins and Molecular Chaperones. Cold Spring Harbor Laboratory, Cold Spring Harbor, pp 335–373
Kiang JG, Tsokos GC 1998 Heat shock proteins 70 kDa: molecular biology, biochemistry and physiology. Pharmacol Ther 80: 183–201
Csermely P, Schnaider T, Söti C, Prohaszka Z, Nardai G 1998 The 90 kDa molecular chaperone family: structure, function, and clinical applications. Pharmacol Ther 79: 129–168
Hammerer-Lercher A, Mair J, Bonatti J, Watzka SB, Puschendorf B, Dirnhofer S 2001 Hypoxia induces heat shock protein expression in human coronary artery bypass grafts. Cardiovasc Res 50: 115–124
David JC, Landry J, Grongnet JF 2001 Perinatal expression of heat-shock protein 27 in brain regions and nonneural tissues of the piglet. J Mol Neurosci 15: 109–120
David JC, Tanguay RM, Grongnet JF 2001 Perinatal expression of HSC70 and HSP70 in neural and non neural tissues of the piglet. Brain Res Dev Brain Res 128: 91–99
David JC, Grongnet JF 2001 Perinatal expression of heat-shock protein 90 in different regions of the brain and in non-neural tissues of the piglet. Biol Neonate 179: 131–139
Kawana K, Miyamoto Y, Tanonaka K, Hanno Y, Yoshida H, Takahashi M, Takeo S 2000 Cytoprotective mechanism of HSP70 against Hypoxia/reoxygenation injury. J Mol Cell Cardiol 32: 2229–2237
Lee JE, Yenari MA, Sun GH, Xu L, Emond MR, Cheng D, Steinberg GK, Giffard RG 2001 Differential neuroprotection from human heat-shock protein 70 overexpression in in vitro and in vivo models of ischemia and ischemia-like conditions. Exp Neurol 170: 129–139
Wenger RH, Gassmann M 1997 Oxygen(es) and the hypoxia inducible factor-1. Biol Chem 378: 609–616
Gassmann M, Wenger RH 1997 HIF-1, a mediator of the molecular response to hypoxia. News Physiol Sci 12: 214–218
Chavez JC, Agani F, Pichiule A, LaManna JC 2000 Expression of hypoxia inducible factor-1α in the brain of rats during chronic hypoxia. J Appl Physiol 89: 1937–1942
Madan A, Varma S, Cohen HJ 2002 Developmental stage-specific expression of the alpha and beta subunits of HIF-1 protein in the mouse and human fetus. Mol Genet Metab 75: 244–249
Bergeron M, Yu AY, Solway KE, Semenza GL, Sharp FR 1999 Induction of hypoxia inducible factor-1 and its target genes following focal ischemia in rat brain. Eur J Neurosci 11: 4159–4170
Katschinski DM, Le L, Heinrich D, Wagner KF, Hofer T, Schindler SG, Wenger RH 2002 Heat induction of the unphosphorylated form of hypoxia inducible factor-1 alpha is dependent on heat-shock protein 90 activity. J Biol Chem 277: 9262–9267
Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC 1998 Dynamic activation of endothelial nitric oxide synthase by HSP90. Nature 392: 821–824
Van Ginneken C, Van Meir F, Sys S, Weyns A 2001 Developmental changes in hemeoxygenase-2 and bNOS expression in enteric neurons in the pig duodenum. Auton Neurosci 91: 16–25
Martin LJ, Brambrink AM, Lehmann C, Portera-Cailliau C, Koehler R, Rothstein J, Traytsman RJ 1997 Hypoxia-ischemia causes abnormalities in glutamate transporters and death of astroglia and neurons in newborn striatum. Ann Neurol 42: 335–348
Ikari A, Nakano M, Kawano K, Suketa Y 2002 Up-regulation of sodium-dependent glucose transporter by interaction with heat shock protein 70. J Biol Chem 277: 33338–33343
Felix B, Leger MF, Albe Fessard D, Marcilloux JC, Rampin O, Laplace JP 1999 Stereotaxic Atlas of the Pig Brain 49. Elsevier, New York, pp 1–137
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ 1952 Protein measurement with the Folin phenol reagent. J Biol Chem 212: 265–275
Conover WJ 1986 Practical Non-Parametric Statistics. Wiley, New York, pp 1–125
Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli KJ, Yuan J, Moskowitz MA 1998 Activation and cleavage of caspase-3 in apoptosis induced by experimental cerebral ischemia. J Neurosci 18: 3659–3668
Taylor DL, Edwards AD, Mehmet H 1999 Oxidative metabolism, apoptosis and perinatal brain injury. Brain Pathol 9: 93–117
Chen J, Jin K, Chen M, Pei W, Kawaguchi K, Greenberg DA, Simon RP 1997 Early detection of DNA strand breaks in the brain after transient focal ischemia: implications for the role of DNA damage in apoptosis and neural cell death. J Neurochem 69: 232–245
Singer D 1999 Review. Neonatal tolerance to hypoxia: a comparative physiological approach. Comp Biochem Physiol A Mol Integr Physiol 123: 221–234
Lutz PC, Hochachka PW 1993 Hypoxia defence mechanisms: a comparison between diving reptiles and mammals. In: Hochachka PW, Lutz PC, Sick T, Rosenthal M, Van den Thillart T (eds) Surviving Hypoxia Mechanisms of Control and Adaptation. CRC Press, Boca Raton, pp 459–469
Ruggins MR 1991 Pathophysiology of apnea in preterm infants. Arch Dis Child 66: 70–73
Diemer K 1968 Capillarisation and oxygen supply of the brain. In: Lübbers DW Luft UC Thews G Witaleb E(eds) Oxygen Transport in Blood and Tissue. Thieme, Stuttgart, pp 118–123
Saugstadt OD 1990 Oxygen toxicity in the neonatal period. Acta Paediatr Scand 79: 881–892
Vannucci RC, Towfighi J, Brucklacher RM, Vannucci SJ 2001 Effect of extreme hypercapnia on hypoxic-ischemic brain damage in the immature rat. Pediatr Res 49: 799–803
Chu D, Qiu J, Grafe M, Fabian R, Kent TA, Rassin D, Nesic O, Werrbach-Perez K, Perez-Polo R 2002 Delayed cell death signalling in traumatized central nervous system: hypoxia. Neurochem Res 27: 97–106
Ikeda T, Abe K, Ota A, Ikenou T 1999 Heat shock protein 70 and heat shock cognate protein 70 messenger ribonucleic acid induction in the brains, hearts, and liver of neonatal rats after hypoxic stress. J Obstet Gynecol 180: 457–481
Xia XY, Ikeda T, Ota A, Xia YX, Sameshima H, Ikenoue T, Toshimori K 1999 Heat shock protein 72 expression and microtubule-associated protein 2 disappearance after hypoxia-ischemia in the developing rat brain. J Obstet Gynecol 180: 1254–1260
Mortola JP 1999 How newborn mammals cope with hypoxia. Respir Physiol 116: 95–103
Fischer DA, Matten J, Reimann J, Bönnemann C, Schröder R 2002 Expression, localization and functional divergence of alphaB-crystallin and heat shock protein 27 in core myopathies and neurogenic atrophy. Acta Neuropathol (Berl) 104: 297–304
Liu Y, Steinacker JM 2001 Changes in skeletal muscle heat shock proteins: pathological significance. Front Biosci 6: D12–D25
Thompson HS, Clarkson PM, Scordilis SP 2002 The repeated bout effect and heat shock proteins: intramuscular HSP27 and HSP70 expression following two bouts of eccentric exercise in humans. Acta Physiol Scand 174: 47–56
Plumier JC, Amstrong JN, Wood NI, Babity JM, Hamilton TC, Hunter AJ, Robertson HA, Currie RW 1997 Differential expression of c-fos, HSP70 and HSP27 after photothrombotic injury in the rat brain. Mol Brain Res 45: 239–246
Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP 2002 HSP27 as a negative regulator of cytochrome c release. Mol Cell Biol 22: 816–834
Sakamoto K, Urushidani T, Nagao T 1998 Translocation of HSP27 to cytoskeleton by repetitive hypoxia-reoxygenation in the rat myoblast cell line, H9C2. Biochem Biophys Res Commun 251: 576–579
Dawson VL, Kizushi VM, Huang PL, Snyder SH, Dawson TM 1996 Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci 16: 2479–2487
Samdani AF, Dawson TM, Dawson VL 1997 Nitric oxide synthase in models of focal ischemia. Stroke 28: 1283–1288
Koh JY, Choi DW 1988 Cultured striatal neurons containing NADPH-diaphorase or acetylcholinesterase are selectively resistant to injury by NMDA receptors. Brain Res 446: 374–378
Agani FH, Puchovicz M, Chavez JC, Pichiule P, LaManna J 2002 Role of nitric oxide in the regulation of HIF-1α expression during hypoxia. Am J Physiol 283: C178–C186
Hudetz AG, Shen H, Kampine JP 1998 Nitric Oxide from neuronal NOS plays critical role in cerebral capillary flow response to hypoxia. Am J Physiol 43: H982–H989
Bellmann K, Burkart V, Bruckhoff J, Kolb H, Landry J 2000 p38 dependent enhancement of cytokine induced nitric oxide synthase gene expression by heat shock protein 70. J Biol Chem 275: 18172–18179
Rossiter JP, Anderson LL, Yang F, Cole GM 2002 Caspase-3 activation and caspase-like proteolytic activity in human perinatal hypoxic ischemic brain injury. Acta Neuropathol (Berl) 103: 66–73
Adachi M, Shoma O, Tsuneishi S, Takada S, Nakamura H 2001 Combination effect of systemic hypothermia and caspase inhibitor administration against hypoxic-ischemic brain damage in neonatal rats. Pediatr Res 50: 590–595
Khurana P, Asraf QM, Misra OP Delivora Papadopoulos M 2002 Effect of hypoxia on caspase-3, -8 and, -9 activity and expression in the cerebral cortex of the newborn piglets. Neurochem Res 27: 931–938
Peeters-Scholte C, Koster J, Veldhuis W, van Den Tweel E, Zhu C, Kops N, Blomgren K, Bär D, Van Buul-Offers S, Hagberg H, Nicolay K, van Bel F, Groenendaal F 2002 Neuroprotection by selective nitric oxide synthase inhibition at 24 hours after perinatal hypoxia-ischemia. Stroke 33: 2304–2310
van den Tweel ER, Peeters Scholte CM, van Bel F, Heijnen CJ, Groenendaal F 2002 Inhibition of nNOS and iNOS following hypoxia-ischemia improves long-term outcome but does not influence the inflammatory response in the neonatal rat. Dev Neurosci 24: 389–395
Calvert JW, Yin W, Patel M, Badr A, Mychaskiw G, Parent AD, Zhang JH 2002 Hyperbaric oxygenation prevented brain injury induced by hypoxia-ischemia in the neonatal rat model. Brain Res 951: 1–8
Acknowledgements
We are grateful to J. Lareynie and M. Lesage for technical assistance and to M. Herrouin for preparing the manuscript. Acknowledgments are also given to Air Liquide for the generous gift of the nitrogen used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
We are grateful to the American Heart Association (grant 0265359T) for one of us (J.C.P.).
Rights and permissions
About this article
Cite this article
Chiral, M., Grongnet, JF., Plumier, JC. et al. Effects of Hypoxia on Stress Proteins in the Piglet Brain at Birth. Pediatr Res 56, 775–782 (2004). https://doi.org/10.1203/01.PDR.0000142732.09325.61
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000142732.09325.61