Abstract
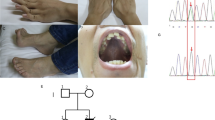
We present here a patient with muscle fatigue and poor growth since the age of 6 y. The diagnosis of a mitochondrial disease was based on the presence of ragged red fibers in the muscle biopsy and on a combined defect of mitochondrial DNA–encoded respiratory enzymes. Epilepsia partialis continua with stroke-like episodes appeared 2 mo before death at the age of 18 and prompted a search for mitochondrial DNA mutations associated with mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes. Minisequencing of the patient's DNA samples revealed a heteroplasmic T3271C mutation with a 78–94% mutation load in her fibroblasts or autopsy-derived tissue samples. This is the ninth reported non-Japanese patient with T3271C mutation. Our patient shows that despite very high proportion of mutant mtDNA, the T3271C mutation can give rise to mild symptoms in childhood and to a rapid terminal phase that simulates encephalitis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- MELAS:
-
mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes
- MERRF:
-
myoclonic epilepsy with ragged red fibers
- MtDNA:
-
mitochondrial DNA
- RRF:
-
ragged red fiber
References
Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP 1984 Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol 16: 481–488
Goto Y, Nonaka I, Horai S 1990 A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature 348: 651–653
Kobayashi Y, Momoi MY, Tominaga K, Momoi T, Nihei K, Yanagisawa M, Kagawa Y, Ohta S 1990 A point mutation in the mitochondrial tRNA(Leu)(UUR) gene in MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes). Biochem Biophys Res Commun 173: 816–822
Tarnopolsky MA, Maguire J, Myint T, Applegarth D, Robinson BH 1998 Clinical, physiological, and histological features in a kindred with the T3271C melas mutation. Muscle Nerve 21: 25–33
Goto Y, Nonaka I, Horai S 1991 A new mtDNA mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS). Biochim Biophys Acta 1097: 238–240
Tokunaga M, Mita S, Sakuta R, Nonaka I, Araki S 1993 Increased mitochondrial DNA in blood vessels and ragged-red fibers in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS). Ann Neurol 33: 275–280
Sakuta R, Goto Y, Horai S, Nonaka I 1993 Mitochondrial DNA mutations at nucleotide positions 3243 and 3271 in mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes: a comparative study. J Neurol Sci 115: 158–160
Yanagawa T, Sakaguchi H, Nakao T, Sasaki H, Matsumoto G, Sanke T, Nanjo K 1998 Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes with deterioration during pregnancy. Intern Med 37: 780–783
Takeda A, Chiba S, Takaaki I, Tanamura A, Yamaguchi Y, Takeda N 1998 Cell cycle of myocytes of cardiac and skeletal muscle in mitochondrial myopathy. Jpn Circ J 62: 695–699
Shinde A, Nakano S, Taguchi Y, Kagawa D, Akiguchi I 2000 A patient of MELAS with 3271 mutation with fatal outcome after alcohol intake. Rinsho Shinkeigaku 40: 561–565
Majamaa K, Moilanen JS, Uimonen S, Remes AM, Salmela PI, Karppa M, Majamaa-Voltti KA, Rusanen H, Sorri M, Peuhkurinen KJ, Hassinen IE 1998 Epidemiology of A3243G, the mutation for mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes: prevalence of the mutation in an adult population. Am J Hum Genet 63: 447–454
Chinnery PF, Johnson MA, Wardell TM, Singh-Kler R, Hayes C, Brown DT, Taylor RW, Bindoff LA, Turnbull DM 2000 The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol 48: 188–193
Ciafaloni E, Ricci E, Shanske S, Moraes CT, Silvestri G, Hirano M, Simonetti S, Angelini C, Donati MA, Garcia C, Martinuzzi A, Mosewich R, Servidei S, Zammarchi E, Bonilla E, DeVivo DC, Rowland LP, Schon E, DiMauro S 1992 MELAS: clinical features, biochemistry, and molecular genetics. Ann Neurol 31: 391–398
van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA 1992 Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet 1: 368–371
Moraes CT, Ciacci F, Silvestri G, Shanske S, Sciacco M, Hirano M, Schon EA, Bonilla E, DiMauro S 1993 Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord 3: 43–50
Hammans SR, Sweeney MG, Hanna MG, Brockington M, Morgan-Hughes JA, Harding AE 1995 The mitochondrial DNA transfer RNALeu(UUR) A–>G(3243) mutation. A clinical and genetic study. Brain 118: 721–734
Morgan-Hughes JA, Sweeney MG, Cooper JM, Hammans SR, Brockington M, Schapira AH, Harding AE, Clark JB 1995 Mitochondrial DNA (mtDNA) diseases: correlation of genotype to phenotype. Biochim Biophys Acta 1271: 135–140
van den Ouweland JM, Maechler P, Wollheim CB, Attardi G, Maassen JA 1999 Functional and morphological abnormalities of mitochondria harbouring the tRNA(Leu)(UUR) mutation in mitochondrial DNA derived from patients with maternally inherited diabetes and deafness (MIDD) and progressive kidney disease. Diabetologia 42: 485–492
Majander A, Rapola J, Sariola H, Suomalainen A, Pohjavuori M, Pihko H 1995 Diagnosis of fatal infantile defects of the mitochondrial respiratory chain: age dependence and postmortem analysis of enzyme activities. J Neurol Sci 134: 95–102
Suomalainen A, Majander A, Haltia M, Somer H, Lonnqvist J, Savontaus ML, Peltonen L 1992 Multiple deletions of mitochondrial DNA in several tissues of a patient with severe retarded depression and familial progressive external ophthalmoplegia. J Clin Invest 90: 61–66
Suomalainen A, Majander A, Pihko H, Peltonen L, Syvanen AC 1993 Quantification of tRNA3243(Leu) point mutation of mitochondrial DNA in MELAS patients and its effects on mitochondrial transcription. Hum Mol Genet 2: 525–534
Shibata DK, Arnheim N, Martin WJ 1988 Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med 167: 225–230
Silvestri G, Bertini E, Servidei S, Rana M, Zachara E, Ricci E, Tonali P 1997 Maternally inherited cardiomyopathy: a new phenotype associated with the A to G AT nt. 3243 of mitochondrial DNA (MELAS mutation). Muscle Nerve 20: 221–225
Huang CC, Chen RS, Chen CM, Wang HS, Lee CC, Pang CY, Hsu HS, Lee HC, Wei YH 1994 MELAS syndrome with mitochondrial tRNA(Leu(UUR)) gene mutation in a Chinese family. J Neurol Neurosurg Psychiatry 57: 586–589
Chen CM, Huang CC 1995 Gonadal dysfunction in mitochondrial encephalomyopathies. Eur Neurol 35: 281–286. (MELAS mutation). Muscle Nerve 20:221–225
Balestri P, Grosso S 2000 Endocrine disorders in two sisters affected by MELAS syndrome. J Child Neurol 15: 755–758
Luoma P, Mehlberg A, Rinnhe JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalinen A 2004 Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study. Lancet 364: 875–882
Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG 2004 Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423
Suzuki T, Koizumi J, Shiraishi H, Ishikawa N, Ofuku K, Sasaki M, Hori T, Ohkoshi N, Anno I 1990 Mitochondrial encephalomyopathy (MELAS) with mental disorder. CT, MRI and SPECT findings. Neuroradiology 32: 74–76
Thomeer EC, Verhoeven WM, van de Vlasakker CJ, Klompenhouwer JL 1998 Psychiatric symptoms in MELAS; a case report. J Neurol Neurosurg Psychiatry 64: 692–693
Chinnery PF, Howell N, Lightowlers RN, Turnbull DM 1997 Molecular pathology of MELAS and MERRF. The relationship between mutation load and clinical phenotypes. Brain 120: 1713–1721
Rahman S, Poulton J, Marchington D, Suomalainen A 2001 Decrease of 3243 A–>G mtDNA mutation from blood in MELAS syndrome: a longitudinal study. Am J Hum Genet 68: 238–240
Makela-Bengs P, Suomalainen A, Majander A, Rapola J, Kalimo H, Nuutila A, Pihko H 1995 Correlation between the clinical symptoms and the proportion of mitochondrial DNA carrying the 8993 point mutation in the NARP syndrome. Pediatr Res 37: 634–639
Silvestri G, Ciafaloni E, Santorelli FM, Shanske S, Servidei S, Graf WD, Sumi M, DiMauro S 1993 Clinical features associated with the A–>G transition at nucleotide 8344 of mtDNA (“MERRF mutation”). Neurology 43: 1200–1206
van den Ouweland JM, Lemkes HH, Gerbitz KD, Maassen JA 1995 Maternally inherited diabetes and deafness (MIDD): a distinct subtype of diabetes associated with a mitochondrial tRNA(Leu)(UUR) gene point mutation. Muscle Nerve 3: S124–130
Taivassalo T, Shoubridge EA, Chen J, Kennaway NG, DiMauro S, Arnold DL, Haller RG 2001 Aerobic conditioning in patients with mitochondrial myopathies: physiological, biochemical, and genetic effects. Ann Neurol 50: 133–141
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by grants from the Alli Paasikivi Foundation and National Graduate School of Clinical Investigation (to L.S.) and the Academy of Finland and Sigrid Juselius Foundation (to A.S.).
Rights and permissions
About this article
Cite this article
Stenqvist, L., Paetau, A., Valanne, L. et al. A Juvenile Case of MELAS with T3271C Mitochondrial DNA Mutation. Pediatr Res 58, 258–262 (2005). https://doi.org/10.1203/01.PDR.0000169966.82325.1A
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/01.PDR.0000169966.82325.1A